1,4-二异硫代氰基丁酯 | 4430-51-7
中文名称
1,4-二异硫代氰基丁酯
中文别名
1,4-二硫异氰酸酯丁烷;1,4-丁烷二硫异氰酸酯
英文名称
1,4-butanediisothiocyanate
英文别名
1,4-diisothiocyanatobutane;1,4-Butane diisothiocyanate
CAS
4430-51-7
化学式
C6H8N2S2
mdl
MFCD00041136
分子量
172.275
InChiKey
RRSISCMPUAGVJN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:30-33°C
-
沸点:170°C 11mm
-
密度:1.17
-
闪点:170°C/11mm
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:88.9
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:6.1
-
危险类别码:R20/21/22,R34
-
危险品运输编号:2928
-
海关编码:2930909090
-
包装等级:II
-
危险类别:6.1
-
安全说明:S26,S36/37/39,S45
-
WGK Germany:3
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis of new antimicrobials. V. Synthesis of alkylenebis-(thiosemicarbazides) and their related compounds.摘要:为了检测其抗微生物活性,合成了几种烷基亚甲基双(硫脲素)和烷基亚甲基双(二硫脲)衍生物。1, 1'-二苄叉-4, 4'-烷基亚甲基双(硫脲素)是通过4, 4'-烷基亚甲基双(硫脲素)和芳醛的反应制备的,而1, 1'-二芳酰-4, 4'-六亚甲基双(硫脲素)则是通过4, 4'-六亚甲基双(硫脲素)与芳酰氯的反应制备的。1, 1'-二烷基或二芳基-6, 6'-烷基亚甲基双(二硫脲)是通过4, 4'-烷基亚甲基双(硫脲素)与烷基或芳基异硫氰酸酯的反应合成的。N, N'-六亚甲基-双[2-氨基-5-(2-甲氧基苯基)噻二唑]是通过1, 1'-双(2-甲氧基苄叉)-4, 4'-六亚甲基双(硫脲素)的环合反应制备的。DOI:10.1248/cpb.23.668
-
作为产物:描述:参考文献:名称:2-氨基-2-脱氧-d-葡萄糖和相关氨基醇作为N-乙酰基-β-d-己糖胺酶抑制剂的脲基和硫脲基衍生物的合成及生物学评价摘要:制备2-乙酰氨基-2-脱氧-β-D-葡萄糖,1-氨基-1-脱氧-D-葡萄糖醇和2-(2-氨基乙氧基)乙醇的脲基和硫脲基衍生物,作为N-乙酰基-β-D-。己糖胺酶(NAHase)抑制剂,并在阴道毛滴虫NAHase上进行了评估。尽管没有一种酶在100 microM时显示出对酶的完全抑制作用,但是1-amino-1-deoxy-D-D-葡萄糖醇衍生物却充当了阴道链球菌NAHase的竞争性抑制剂。DOI:10.1016/s0008-6215(98)00286-9
文献信息
-
Synthesis of new antimicrobials. IV. Synthesis of alkylenebis-(thiourea) derivatives and their related compounds.作者:TAKAHIRO YABUUCHI、MASAKATU HISAKI、MASAHIRO MATUDA、RYUICHI KIMURADOI:10.1248/cpb.23.663日期:——In order to examine the antimicrobial activity, alkylenebis (thiourea) derivatives and their related compounds were synthesized. 3, 3'-Substituted 1, 1'-alkylene-bis-(thiourea) derivatives were prepared from alkylenebis (isothiocyanates) and amines. Also 3, 3'-alkylene-bis [2-thio-2, 4 (1H, 3H)-quinazolinediones] were prepared by the reaction of alkylenebis (isothiocyanates) and anthranilic acid, or alkylene diamines and ethyl o-isothiocyanatobenzoate respectively.
-
BIOOCOMPATIBLE POLYMER COMPOSITIONS申请人:Gunatillake Pathiraja Arachchillage公开号:US20090324675A1公开(公告)日:2009-12-31The present invention provides a biocompatible prepolymer comprising hydrophilic and hydrophobic segments, wherein the hydrophobic segments have at least one ethylenically unsaturated functional group and at least 5% of the segments have two or more ethylenically unsaturated functional groups and water. The invention further provides a biocompatible prepolymer composition comprising hydrophilic and hydrophobic prepolymers, wherein at least one of the hydrophobic prepolymers has at least one ethylenically unsaturated functional group and at least 5% of the prepolymers have two or more ethylenically unsaturated functional groups and water. The invention further provides use of the prepolymer or prepolymer compositions of the invention in biomedical applications such as tissue engineering, as bone substitutes or scaffolds, and in wound treatment.
-
Synthesis and Antispasmodic Activity Evaluation of Bis-(Papaverine) Analogues作者:Jaskiran Kaur、Narendra Nath Ghosh、Ramesh ChandraDOI:10.1248/cpb.52.316日期:——bis-(tetrahydropapaverine) ring systems have been synthesised in expectation of better antispasmodic activity in comparison with papaverine. The synthesis of the targeted heterocycles is described along with a discussion of their structure activity relationship. The general synthetic methods of bis-(tetrahydropapaverine) analogues involve tetrahydropapaverine, various piperazines, diisocyanates and diisothiocyanates
-
[EN] MACROCYCLIC BENZOFURAN AND AZABENZOFURAN COMPOUNDS FOR THE TREATMENT OF HEPATITIS C<br/>[FR] COMPOSÉS BENZOFURANE ET AZABENZOFURANE MACROCYCLIQUES POUR LE TRAITEMENT DE L'HÉPATITE C申请人:BRISTOL MYERS SQUIBB CO公开号:WO2014110066A1公开(公告)日:2014-07-17Compounds of formula I, including their salts, as well as compositions and methods of using the compounds are set forth. The compounds have activity against hepatitis C virus (HCV) and may be useful in treating those infected with HCV.化合物I的化合物,包括它们的盐,以及使用这些化合物的组合物和方法。这些化合物对丙型肝炎病毒(HCV)具有活性,可能对感染HCV的人有用。
-
CHROMENE COMPOUND, CURABLE COMPOSITION COMPRISING THE COMPOUND, AND OPTICAL ARTICLE INCLUDING A CURED BODY OF THE CURABLE COMPOSITION申请人:TOKUYAMA CORPORATION公开号:US20200190106A1公开(公告)日:2020-06-18A chromene compound having at least one indenonaphthopyran moiety which has a group forming a spiro ring together with the 13-position carbon atom and further an oligomer chain group selected from a polyalkylene oxide oligomer chain group having at least three recurring units and a polyester oligomer chain group having at least three recurring units, represented by the following formula and having reduced matrix dependence: wherein R 1 and R 2 are each a group which may have an oligomer chain group, the ring Z bonded to the 13-position carbon atom of the chromene compound is a Spiro ring group, and R 3 and R 4 are each an aryl group or heteroaryl group which may have an oligomer chain group. Preferably, the chromene compound has at least one oligomer chain group in the molecule.
表征谱图
-
氢谱1HNMR
-
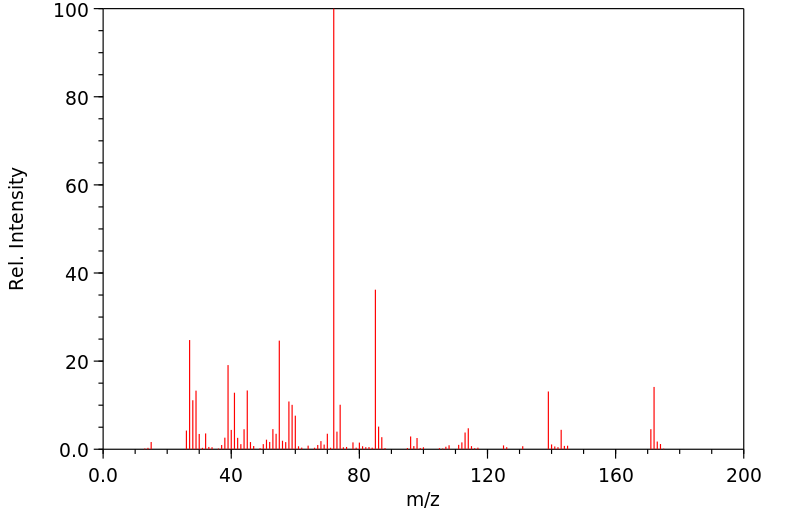
质谱MS
-
碳谱13CNMR
-
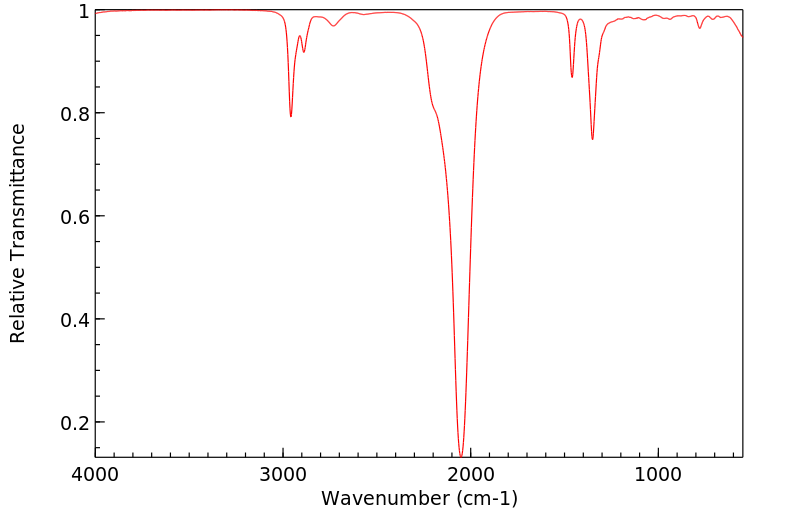
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-异硫氰基-1-环戊羧酸乙酯
顺-2-异硫氰基-1-环己烷羧酸乙酯
羰基异氰酸酯异硫氰酸酯
羰基二异硫氰酸酯
硫代异氰酸环戊酯
甲代烯丙基异硫氰酸酯
环己烷羰基异硫氰酸酯
环己基异硫氰酸脂
环丙基甲基异硫氰酸酯
环丁烷羰基异硫氰酸酯
异硫氰酸甲酯
异硫氰酸甲氧基甲酯
异硫氰酸甲基环己酯
异硫氰酸环辛酯
异硫氰酸环庚脂
异硫氰酸环十二酯
异硫氰酸环丙酯
异硫氰酸烯丙酯
异硫氰酸溴代乙酯
异硫氰酸氯代乙酯
异硫氰酸氨基甲酰
异硫氰酸异戊酯
异硫氰酸异丙酯
异硫氰酸异丁酯
异硫氰酸己酯
异硫氰酸叔辛酯
异硫氰酸十六酯
异硫氰酸十一烷酯
异硫氰酸仲丁酯
异硫氰酸乙酰酯
异硫氰酸乙酯
异硫氰酸乙烯
异硫氰酸3-丁烯酯
异硫氰酸2-甲氧基乙酯
异硫氰酸1-金刚烷酯
异硫氰酰甲酸甲酯
异硫氰酰甲酸乙酯
异硫氰基环丁烷
异硫代氰酰基乙醛二甲基乙缩醛
异氰酸丙酯
己烷,1-[(2-异硫氰基乙基)硫代]-
己烯,1-异硫氰基-
天然芥菜籽油
叔戊基异硫氰酸酯
叔丁基异硫氰酸酯
双(1-异硫氰基乙基)醚
十八烷基异氰酸酯
二(异硫氰酰甲基)醚
丙脒,N-丁基-2-氯-
三芥子酸甘油酯