1,5-二甲基四唑 | 5144-11-6
中文名称
1,5-二甲基四唑
中文别名
——
英文名称
1,5-dimethyl-1H-tetrazole
英文别名
1,5-dimethyltetrazole;1,5-Dimethyltetrazol
CAS
5144-11-6
化学式
C3H6N4
mdl
MFCD00190183
分子量
98.1074
InChiKey
HWHNFJYQDMSYAF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:70-71 °C
-
沸点:173.61°C (rough estimate)
-
密度:1.0852 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):0
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:43.6
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2933990090
-
储存条件:储存条件:2-8°C,避光,惰性气体环境下保存。
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-乙基-1-甲基-1H-四唑 5-ethyl-1-methyl-1H-tetrazole 90329-50-3 C4H8N4 112.134
反应信息
-
作为反应物:参考文献:名称:DAVIES, R. V.;GREEN, R. D.摘要:DOI:
-
作为产物:参考文献:名称:DE545850摘要:公开号:
-
作为试剂:参考文献:名称:Thuong, Nguyen Thanh; Barbier, Christine; Asseline, Ulysse, Phosphorus and Sulfur and the Related Elements, 1983, vol. 14, p. 357 - 366摘要:DOI:
文献信息
-
A facile and convenient synthesis of substituted tetrazole derivatives from ketones or α,β-unsaturated ketones作者:Abdel-Aziz S. El-Ahl、Saad S. Elmorsy、Hanan Soliman、Fathy A. AmerDOI:10.1016/0040-4039(95)01513-h日期:1995.10Triazidochlorosilane (SiCl4 - NaN3 in situ) is a new and efficient reagent for the direct conversion of ketones or α,β-unsaturated ketones to the corresponding tetrazole derivatives in nearly quantitative yield.
-
Intermediates for the preparation of antihypercholesterolemic tetrazole申请人:Bristol-Myers Company公开号:US04898949A1公开(公告)日:1990-02-06This invention provides novel tetrazole intermediates of the formula ##STR1## wherein R.sup.1 and R.sup.4 each are independently hydrogen, halogen, C.sub.1-4 alkyl, C.sub.1-4 alkoxy or trifluoromethyl; R.sup.2,R.sup.3,R.sup.5 and R.sup.6 each are independently hydrogen, halogen, C.sub.1-4 alkyl or C.sub.1-4 alkoxy; B is hydrogen, C.sub.1-4 alkoxycarbonyl, CH.sub.2 Y or CH.sub.2 Z; Y is hydrogen, hydroxyl or X; Z is ##STR2## X is bromo, chloro or iodo; R.sup.10 is C.sub.1-4 alkyl; and R.sup.11 is phenyl which is unsubstituted or substituted by one or two C.sub.1-4 alkyl or chloro substituents. and processes thereof which are useful for the preparation of antihypercholesterolemic agents.这项发明提供了新型的四唑中间体,其化学式为##STR1##其中R.sup.1和R.sup.4分别独立地是氢、卤素、C.sub.1-4烷基、C.sub.1-4烷氧基或三氟甲基;R.sup.2、R.sup.3、R.sup.5和R.sup.6分别独立地是氢、卤素、C.sub.1-4烷基或C.sub.1-4烷氧基;B是氢、C.sub.1-4烷氧羰基、CH.sub.2 Y或CH.sub.2 Z;Y是氢、羟基或X;Z是##STR2##X是溴、氯或碘;R.sup.10是C.sub.1-4烷基;R.sup.11是苯基,未取代或取代一个或两个C.sub.1-4烷基或氯代基。以及用于制备抗高胆固醇药物的相关方法。
-
Antihypercholesterolemic tetrazole compounds申请人:Bristol-Meyers Company公开号:US04897490A1公开(公告)日:1990-01-30Compounds of the formula ##STR1## wherein R.sup.1 and R.sup.4 each are independently hydrogen, halogen, C.sub.1-4 alkyl, C.sub.1-4 alkoxy, or trifluoromethyl; R.sup.2, R.sup.3, R.sup.5 and R.sup.6 each are independently hydrogen, halogen C.sub.1-4 alkyl or C.sub.1-4 alkoxy; tet is ##STR2## n is an integer of from 0 to 2, inclusive; A is ##STR3## R.sup.7 is hydrogen, C.sub.1-4 alkyl, C.sub.1-4 alkoxy(lower) alkyl or (2-methoxyethoxy)methyl; X is --OH or .dbd.O; and R.sup.8 is hydrogen, a hydrolyzable ester group or a cation to form a non-toxic pharmaceutically acceptable salt, are novel antihypercholesterolemic agents which inhibit cholesterol biosynthesis. Intermediates and processes for their preparation are disclosed.式为##STR1##的化合物,其中R.sup.1和R.sup.4分别独立地是氢、卤素、C.sub.1-4烷基、C.sub.1-4氧烷基或三氟甲基;R.sup.2、R.sup.3、R.sup.5和R.sup.6分别独立地是氢、卤素、C.sub.1-4烷基或C.sub.1-4氧烷基;tet为##STR2##;n是0到2之间的整数;A为##STR3##;R.sup.7为氢、C.sub.1-4烷基、C.sub.1-4氧烷基(较低)烷基或(2-甲氧基乙氧基)甲基;X为--OH或.dbd.O;R.sup.8为氢、可水解酯基团或阳离子,以形成一种无毒的药用可接受盐,这些化合物是新型抗高胆固醇药物,可抑制胆固醇生物合成。揭示了它们的中间体和制备方法。
-
Antihypertensive 3-tetrazoyl-4-quinolones申请人:The Boots Company Plc公开号:US04659718A1公开(公告)日:1987-04-21Novel quinolones of formula I ##STR1## and pharmaceutically acceptable acid addition salts thereof in which the dotted line between positions 2 and 3 of the quinolone ring represents an optional bond, R is hydrogen, 1-methyl or 2-lower alkyl; R.sub.1 is lower alkyl; R.sub.2 is hydrogen, halo, lower alkyl, lower alkoxy, trifluoromethyl, cyano, difluoromethoxy, methylsulphinyl, phenylsulphinyl or the group --NR.sub.4 R.sub.5 or the N-oxide thereof wherein R.sub.4 and R.sub.5, which may be the same or different, are lower alkyl or, together with the nitrogen atom to which they are attached form a pyrrolidino, piperidino or morpholino radical; and R.sub.3 is hydrogen, fluoro, lower alkyl or lower alkoxy provided that, when R.sub.3 is lower alkoxy, R.sub.2 is other than lower alkoxy have utility as antihypertensive agents. Processes for preparing the quinolones and pharmaceutical compositions containing them are disclosed.
-
Reaction of Trimethylsilyl Azide with C=N–O Bond作者:Kozaburo Nishiyama、Izumi MiyataDOI:10.1246/bcsj.58.2419日期:1985.8Trimethylsilyl azide (TMSA) was reacted with an oxime ester or a Reissert salt in the presence of trimethylsilyl trifluoromethanesulfonate to give tetrazole derivative. The details of these reactions are examined.
表征谱图
-
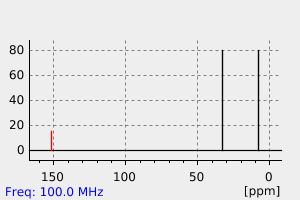
氢谱1HNMR
-
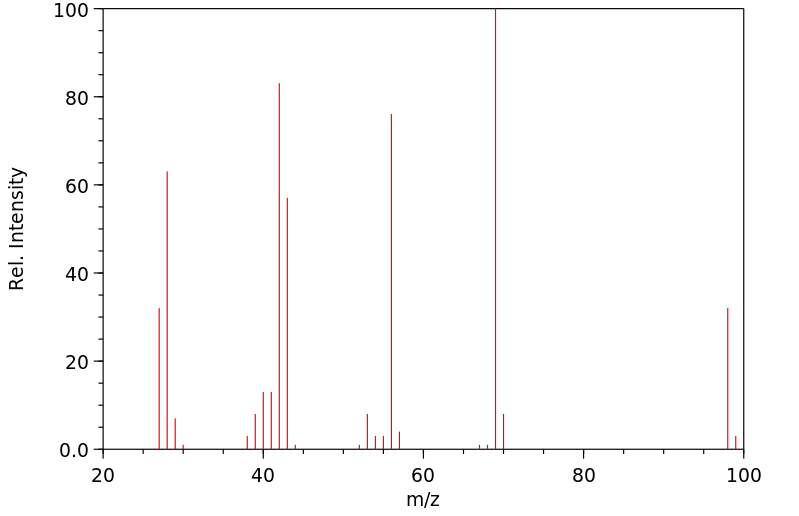
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)