1,8-二氯-9,10-双(苯乙炔基)蒽 | 51749-83-8
中文名称
1,8-二氯-9,10-双(苯乙炔基)蒽
中文别名
1,8-二氯-9,10-二苯乙炔基蒽;1,8-二氯-9,10-二苯乙炔基蒽(DBEA);1,8-二氯-9,10-双苯乙炔蒽;1,8-二氯-9,1-二苯乙炔基蒽;DBEA
英文名称
1,8-dichloro-9,10-bis(phenylethynyl)anthracene
英文别名
1,8-dichloro-9,10-bis(2-phenylethynyl)anthracene
CAS
51749-83-8
化学式
C30H16Cl2
mdl
MFCD00012048
分子量
447.363
InChiKey
YONGNHJIWAYNLC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:168-170 °C(lit.)
-
沸点:524.89°C (rough estimate)
-
密度:1.0703 (rough estimate)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):9.7
-
重原子数:32
-
可旋转键数:4
-
环数:5.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险品标志:Xn
-
危险类别码:R40,R36/37/38,R20/21/22
-
海关编码:2903999090
-
安全说明:S26,S36/37/39,S45
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
反应信息
-
作为反应物:描述:4-methoxy-N-(4-methoxyphenyl)-N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)aniline 、 1,8-二氯-9,10-双(苯乙炔基)蒽 在 四(三苯基膦)钯 、 potassium carbonate 作用下, 以 乙醇 、 水 、 甲苯 为溶剂, 以66 %的产率得到参考文献:名称:CN115850098摘要:公开号:
-
作为产物:描述:1,8-dichloro-9,10-bis(phenylethynyl)-9,10-dihydroanthracene-9,10-diol 在 tin(ll) chloride 作用下, 以 溶剂黄146 为溶剂, 以37%的产率得到1,8-二氯-9,10-双(苯乙炔基)蒽参考文献:名称:Hanhela, Peter J.; Paul, Brenton D., Australian Journal of Chemistry, 1981, vol. 34, # 8, p. 1701 - 1717摘要:DOI:
文献信息
-
Reagents and methods for direct labeling of nucleotides申请人:Naleway John J.公开号:US20130150254A1公开(公告)日:2013-06-13The present invention provides systems and methods for production of activatable diazo-derivatives for use in labeling nucleotides. Labeling nucleotides is accomplished by contacting a stable hydrazide derivative of a detectable moiety with an activating polymer reagent which is used to directly label the nucleotide sample. Labeling occurs on the phosphate backbone of the nucleotide which does not perturb hybridization of the labeled nucleotide with its anti-sense strand. Since the method involves direct labeling, all types of nucleotides can be labeled without prior amplification or alteration.
-
Blue/violet diphenylanthracene chemiluminescent fluorescers申请人:Cranor Earl公开号:US20100327240A1公开(公告)日:2010-12-30Compounds are disclosed for the production of chemiluminescent light, particularly for the production of blue/violet light within the range of about 390 nm to less than 438 nm, and most particularly to the use of compounds composed of symmetrically and asymmetrically substituted anthracenes which are effective for increasing the production of such blue/violet light when used as fluorescers in conjunction with chemiluminescent systems. These systems utilize derivatives of 9,10-diphenylanthracene containing one or more fluorines As shown in General Formulae 1-3. The variables shown in Formulae 1-3 are defined in the specification.
-
Method for producing aqueous polymer dispersions containing colorants申请人:Mathauer Klemens公开号:US20050075453A1公开(公告)日:2005-04-07The present invention relates to a process for preparing dye-comprising aqueous polymer dispersions by free-radical aqueous emulsion polymerization of ethylenically unsaturated monomers in the presence of free-radical initiators, in which at least some of the monomers are employed in the form of an oil-in-water emulsion E1 whose disperse phase comprises at least one oil-soluble dye, wherein the disperse phase of E1 is formed essentially of dye-comprising monomer droplets having a diameter<500 nm. The present invention also relates to perylene dyes of the formula III as set forth in claim 35. The present invention relates in addition to dye-comprising formulations which comprise the dye-comprising polymers of the invention and to pigmented formulations which comprise a polymer of the invention comprising optical brightener.
-
REAGENTS AND METHODS FOR DIRECT LABELING OF NUCLEOTIDES申请人:MARKER GENE TECHNOLOGIES, INC.公开号:US20150152476A1公开(公告)日:2015-06-04The present invention provides systems and methods for production of activatable diazo-derivatives for use in labeling nucleotides. Labeling nucleotides is accomplished by contacting a stable hydrazide derivative of a detectable moiety with an activating polymer reagent which is used to directly label the nucleotide sample. Labeling occurs on the phosphate backbone of the nucleotide which does not perturb hybridization of the labeled nucleotide with its anti-sense strand. Since the method involves direct labeling, all types of nucleotides can be labeled without prior amplification or alteration.
-
A method for protecting the liquid components of a chemiluminescent system and a chemiluminescent light-generating system thus protected申请人:AMERICAN CYANAMID COMPANY公开号:EP0011911A1公开(公告)日:1980-06-11In a chemiluminescent lighting system having separated components in storage before use, at least one of the separate components is stored in a container made from a laminate of polypropylene film and aluminium foil. Good stability of both oxalate ester components and hydroperoxide components when stored in such containers is demonstrated.
表征谱图
-
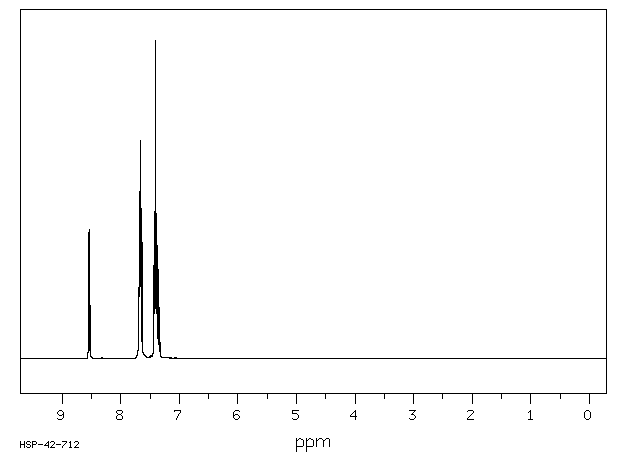
氢谱1HNMR
-
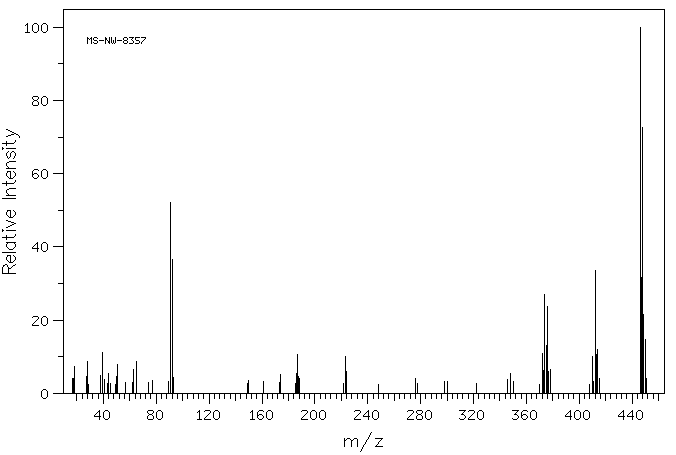
质谱MS
-
碳谱13CNMR
-
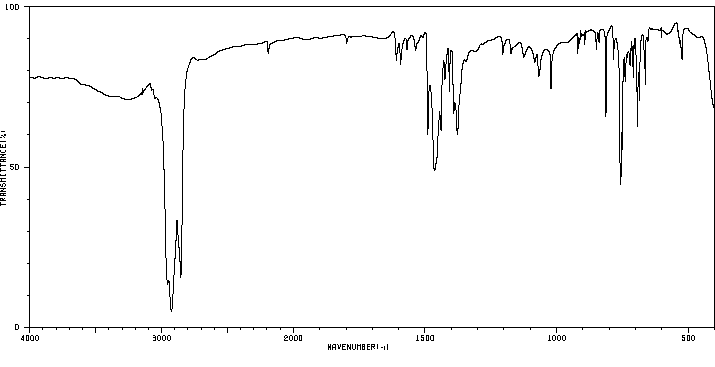
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62