1-环己基-3-苯基-2-硫脲 | 722-03-2
中文名称
1-环己基-3-苯基-2-硫脲
中文别名
——
英文名称
N-cyclohexyl-N'-phenylthiourea
英文别名
1-cyclohexyl-3-phenylthiourea;N-Phenyl-N'-cyclohexyl-thioharnstoff;1-cyclohexyl-3-phenyl-2-thiourea
CAS
722-03-2
化学式
C13H18N2S
mdl
MFCD00021317
分子量
234.365
InChiKey
PASBFQMDQRGJBH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:148 °C
-
沸点:346.1±25.0 °C(Predicted)
-
密度:1.12±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.461
-
拓扑面积:56.2
-
氢给体数:2
-
氢受体数:1
安全信息
-
包装等级:III
-
危险类别:6.1
-
危险性防范说明:P264,P270,P301+P310,P405,P501
-
危险品运输编号:2811
-
危险性描述:H301
-
储存条件:存储条件:2-8°C,干燥。
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-烯丙基-3-苯基-2-硫脲 1-allyl-3-phenyl-thiourea 7341-63-1 C10H12N2S 192.285 1-环己基-3-苯基脲 N-cyclohexyl-N'-phenylurea 886-59-9 C13H18N2O 218.299
反应信息
-
作为反应物:描述:1-环己基-3-苯基-2-硫脲 以98.9%的产率得到N-cyclohexyl-2-benzothiazolamine参考文献:名称:Process for the manufacture of 2-amino-aryleno-thiazole compounds and of摘要:2-氨基芳基噻唑,其中2-位置的氨基团可以被芳基、烷基和/或环烷基取代,以及在环氮原子上被芳基、烷基或环烷基取代的2-亚氨基芳基噻唑烷通过携带相应取代基的芳基硫脲的环化产生,使用亚硫酰氯作为环化剂。改进工艺的优点在于生成的硫量非常低,其他副产物易于分离并可进一步使用。这些噻唑和噻唑烷以高产率和纯度获得。它们是有价值的起始化合物,特别适用于染料的制造。公开号:US04252963A1
-
作为产物:描述:N,N'-二环己基硫脲 在 glassy phosphoric acid 作用下, 生成 1-环己基-3-苯基-2-硫脲参考文献:名称:Skita; Rolfes, Chemische Berichte, 1920, vol. 53, p. 1255摘要:DOI:
文献信息
-
Cerium ammonium nitrate-catalyzed aerobic oxidative coupling of dithiocarbamates: facile synthesis of thioureas and bis(aminothiocarbonyl)disulfides作者:Tian-Tian Li、Xiang-Hai Song、Mei-Shuang Wang、Ning MaDOI:10.1039/c4ra04628e日期:——Diverse disubstituted and trisubstituted thioureas were synthesized by the condensation of dithiocarbamate TEA (or DABCO) salts and amines using cerium ammonium nitrate (CAN) as a catalyst in high yields at room temperature. It is a one-pot method and it is unnecessary to isolate isothiocyanates. This reaction probably took place through nucleophilic addition of amines to isothiocyanates, which were generated by oxidative coupling of dithiocarbamates and the following decomposition of bis(aminothiocarbonyl)disulfides. When secondary amines and CS2 served as the reactants, bis(aminothiocarbonyl)disulfides were obtained via tandem nucleophilic addition/oxidative coupling reactions in moderate to excellent yields. In all the coupling reactions, the oxidant was air and CAN possibly acted as an SET catalyst.
-
Synthetic Studies toward Aryl-(4-aryl-4<i>H</i>-[1,2,4]triazole-3-yl)-amine from 1,3-Diarylthiourea as Urea Mimetics作者:Amarnath Natarajan、Yuhong Guo、Haribabu Arthanari、Gerhard Wagner、Jose A. Halperin、Michael ChorevDOI:10.1021/jo0508189日期:2005.8.14]triazole-3-yl-amines as urea mimetics from the corresponding 1,3-disubstituted thioureas has been studied, and the scope and limitations of this reaction are presented. The reaction proceeds through the formation of a carbodiimide, followed by a sequential addition−dehydration with acyl hydrazides. 1,3-Branched dialkylthioureas result in the formation of the corresponding ureas. The electronic and steric
-
A New Synthetic Protocol for the Preparation of Carbodiimides Using a Hypervalent Iodine(III) Reagent作者:Yunyang Wei、Chenjie Zhu、Dan XuDOI:10.1055/s-0030-1258414日期:2011.3A new, simple, and efficient preparation of symmetrical and unsymmetrical carbodiimides from the corresponding thioureas via dehydrosulfurization using a hypervalent iodine(III) reagent is described. The oxidation afforded carbodiimides in excellent yields and high selectivity. A possible mechanism for the transformation is proposed. desulfurization - hypervalent iodine - oxidations - carbodiimides
-
Nickel Catalysis Enables Access to Thiazolidines from Thioureas via Oxidative Double Isocyanide Insertion Reactions作者:Wen-Kui Yuan、Yan Fang Liu、Zhenggang Lan、Li-Rong Wen、Ming LiDOI:10.1021/acs.orglett.8b03098日期:2018.11.16Ni-catalyzed oxidative double isocyanide insertion to thioureas under air conditions, in which thioureas play three roles as a substrate, a ligand, and overcoming isocyanide polymerization. The reaction is featured by employing a low-cost and low loading Ni(acac)2 catalyst, without any additives, and high atom economy. This is the first example to directly apply a Ni(II) catalyst in oxidative double isocyanide
-
o-Iodoxybenzoic Acid Mediated Oxidative Desulfurization of 1,3-Disubstituted Thioureas to Carbodiimides作者:Krishnacharya Akamanchi、Pramod Chaudhari、Prasad DangateDOI:10.1055/s-0030-1259072日期:2010.12An efficient and mild oxidative desulfurization procedure using o-iodoxybenzoic acid has been developed for the synthesis of carbodiimides starting from easily synthesizable 1,3-disubstituted thioureas.
表征谱图
-
氢谱1HNMR
-
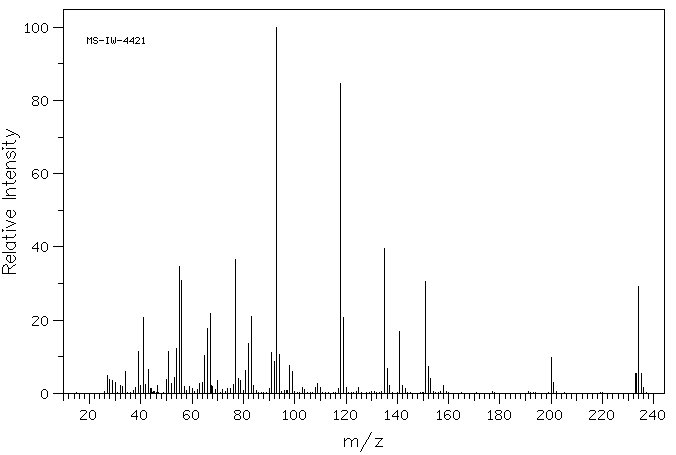
质谱MS
-
碳谱13CNMR
-
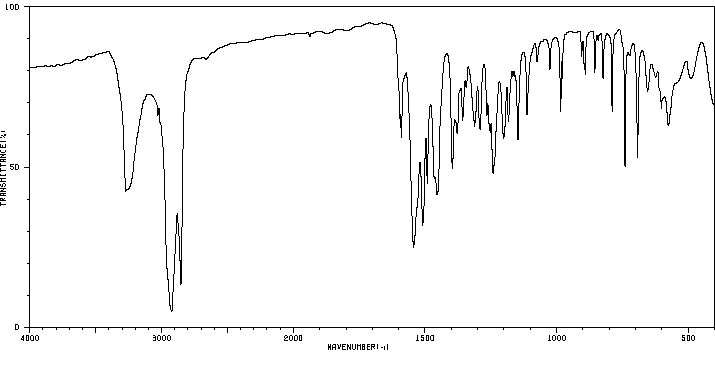
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫