氨基硫脲单盐酸盐 | 4346-94-5
中文名称
氨基硫脲单盐酸盐
中文别名
氨基硫脲盐酸盐
英文名称
thiosemicarbazide hydrochloride
英文别名
Aminothiourea;hydron;chloride
CAS
4346-94-5
化学式
CH6N3S*Cl
mdl
MFCD00060206
分子量
127.598
InChiKey
LEHOGBUBQSGCOK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:190-191 °C (decomp)
-
稳定性/保质期:
常温常压下稳定,为白色结晶。
计算性质
-
辛醇/水分配系数(LogP):-0.77
-
重原子数:6
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:96.2
-
氢给体数:4
-
氢受体数:2
安全信息
-
危险等级:6.1
-
RTECS号:VT4300000
-
海关编码:2930909090
-
包装等级:III
-
危险类别:6.1
-
危险性防范说明:P264,P270,P273,P301+P310+P330,P405,P501
-
危险品运输编号:2811
-
危险性描述:H301,H412
-
储存条件:常温、避光、通风干燥处,密封保存。
SDS
氨基硫脲盐酸盐 修改号码:5
模块 1. 化学品
产品名称: ThiosemicarBAzide Hydrochloride
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第3级
环境危害
急性水生毒性 第3级
慢性水生毒性 第3级
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吞咽会中毒。
对水生生物有害
长期影响对水生生物有害
防范说明
[预防] 避免释放到环境中。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
[急救措施] 食入:立即呼叫解毒中心/医生。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 氨基硫脲盐酸盐
百分比: >98.0%(T)
CAS编码: 4346-94-5
氨基硫脲盐酸盐 修改号码:5
模块 3. 成分/组成信息
分子式:
CH5N3S·HCl
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 一旦发生火警,有爆炸的危险。由于爆炸的危险,远距离灭火。
小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。切勿引起泄漏、溢出或分散。切勿产
生不必要的蒸气。远离热源/火花/明火/热表面。禁烟。采取措施防止静电积累。使用
防爆设备。避免冲击和摩擦。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
确保容器不会受到未测影响,比如跌落或脱落。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
氨基硫脲盐酸盐 修改号码:5
模块 8. 接触控制和个体防护
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 溶于
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 热、撞击、摩擦等条件下可能爆炸分解。
避免接触的条件: 热, 冲击, 摩擦
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物, 氯化氢
模块 11. 毒理学信息
急性毒性: orl-rat LD:>10 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: VT4300000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 84.6 % (by BOD), 93 % (by TOC), 100 % (by GC)
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
氨基硫脲盐酸盐 修改号码:5
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门和专家。建议在可燃溶剂中溶解混合,然后在装有后燃和洗涤装置的化
学焚烧炉中慢慢焚烧。如果一次性焚烧大量物质,可能发生爆炸。废弃处置时请遵守国家、地区和当地的所有法规
。
模块 14. 运输信息
联合国分类: 第1项 毒害品。
UN编号: 2811
正式运输名称: 有毒固体, 有机物, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: ThiosemicarBAzide Hydrochloride
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第3级
环境危害
急性水生毒性 第3级
慢性水生毒性 第3级
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吞咽会中毒。
对水生生物有害
长期影响对水生生物有害
防范说明
[预防] 避免释放到环境中。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
[急救措施] 食入:立即呼叫解毒中心/医生。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 氨基硫脲盐酸盐
百分比: >98.0%(T)
CAS编码: 4346-94-5
氨基硫脲盐酸盐 修改号码:5
模块 3. 成分/组成信息
分子式:
CH5N3S·HCl
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 一旦发生火警,有爆炸的危险。由于爆炸的危险,远距离灭火。
小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。切勿引起泄漏、溢出或分散。切勿产
生不必要的蒸气。远离热源/火花/明火/热表面。禁烟。采取措施防止静电积累。使用
防爆设备。避免冲击和摩擦。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
确保容器不会受到未测影响,比如跌落或脱落。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
氨基硫脲盐酸盐 修改号码:5
模块 8. 接触控制和个体防护
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 溶于
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 热、撞击、摩擦等条件下可能爆炸分解。
避免接触的条件: 热, 冲击, 摩擦
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物, 氯化氢
模块 11. 毒理学信息
急性毒性: orl-rat LD:>10 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: VT4300000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 84.6 % (by BOD), 93 % (by TOC), 100 % (by GC)
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
氨基硫脲盐酸盐 修改号码:5
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门和专家。建议在可燃溶剂中溶解混合,然后在装有后燃和洗涤装置的化
学焚烧炉中慢慢焚烧。如果一次性焚烧大量物质,可能发生爆炸。废弃处置时请遵守国家、地区和当地的所有法规
。
模块 14. 运输信息
联合国分类: 第1项 毒害品。
UN编号: 2811
正式运输名称: 有毒固体, 有机物, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:氨基硫脲单盐酸盐 在 sodium pyruvate 、 calcium carbonate 作用下, 以 水 为溶剂, 以55 %的产率得到(2E)-2-(carbamothioylhydrazinylidene)propanoic acid参考文献:名称:Introducing a novel crystal form of pyruvic acid thiosemicarbazone and its sodium salt摘要:The reaction of thiosemicarbazide and sodium pyruvate has been thoroughly studied and the novel crystal form of pyruvic acid thiosemicarbazone (H2pt) and its sodium salt were obtained. Compounds were characterized by IR spectra, melting points, elemental analysis and conductometric measurements, as well as single-crystal X-ray analysis. A detailed comparative analysis of crystal structures of these compounds is given, as well as comparison with some of the earlier known complexes containing H2pt. The two novel crystal structures exhibit notably different hydrogen bonding patterns, mutually and in comparison with previously reported crystal form of H2pt. All crystal structures are stabilized by extensive network of N-H...O, O-H...O and N-H...S hydrogen bonds. The cyclic hydrogen bonding motif involving the thioureido moieties of the ligand is the only one which repeats in each structure.DOI:10.2298/jsc240417050b
-
作为产物:参考文献:名称:硫代氨基脲盐酸盐的生长和表征:一种半有机NLO材料摘要:硫代氨基脲与盐酸盐在双蒸水中以1:1的摩尔比混合,合成了硫代氨基脲盐酸盐(TSCHCL)。TSCHCL的单晶在室温下通过缓慢蒸发生长,并通过单晶X射线衍射研究确定分子结构,并通过FT-IR,1 H和13表征13 C NMR光谱分析证实合成的化合物。热重分析和差热分析揭示了晶体的热稳定性。TSCHCL的透射光谱表明,晶体在380-1100 nm的波长范围内是透明的。高分辨率X射线衍射法(HRXRD)用于评估生长晶体的完善性。使用维氏显微硬度测试研究了生长晶体的机械性能。使用Nd:YAG激光测试了粉状TSCHCL的二次谐波产生效率,约为正磷酸二氢钾的1.5倍。DOI:10.1016/j.saa.2011.07.007
-
作为试剂:描述:3,5-二叔丁基水杨醛 在 水 、 氨基硫脲单盐酸盐 、 三乙胺 作用下, 以 乙醇 为溶剂, 反应 8.0h, 以85%的产率得到N,N'-bis(3,5-di-tert-butylsalicylidene)hydrazine参考文献:名称:改性位阻酚的合成及抗氧化活性摘要:合成了含有位阻酚片段的水杨醛衍生物。获得的化合物能够提供抗紫外线辐射的保护。DOI:10.1134/s1070363216030166
文献信息
-
Structure–activity relationship study of thiosemicarbazones on an African trypanosome: Trypanosoma brucei brucei作者:Houssou Raymond Fatondji、Salomé Kpoviessi、Fernand Gbaguidi、Joanne Bero、Veronique Hannaert、Joëlle Quetin-Leclercq、Jacques Poupaert、Mansourou Moudachirou、Georges Coffi AccrombessiDOI:10.1007/s00044-012-0208-6日期:2013.5structures. This study that was done using acetophenone thiosemicarbazone (1) as basic model, showed that: (a) the presence of lipophilic substituents in para position on benzene ring, (b) substitution of benzene ring and (c) substitution of hydrogen of thioamide function by a phenyl, strongly influence trypanocidal activity. The various modifications to basic structure (1) allowed the synthesis of 1-(4-chlorophenyl)探索非洲锥虫缩氨基硫脲的结构-活性关系:布氏锥虫布氏,一系列的35缩氨基硫脲(1 - 35)已被合成和表征可以通过1 H NMR,13 C NMR,和FT-IR光谱。使用“ Lilit alamar blue”方法测试所有化合物的杀锥虫活性。考虑到它们的结构,比较了硫半脲的锥虫杀菌能力。该研究结果表明,使用苯乙酮缩氨基硫脲进行(1)作为基本模型,表明:(a)中的亲脂性的取代基的存在对在苯环上的位置,(b)苯环的取代和(c)硫代酰胺官能团的氢被苯基取代,强烈影响锥虫活性。对基本结构(1)的各种修饰允许合成1-(4-氯苯基)亚乙基-4-苯基-硫代氨基脲(34)。该化合物具有3.97μM的锥虫杀灭活性,是该系列中最活跃的。
-
Unsaturated lactones. LXXIII. Synthesis and antiviral activity of some substituted 2-buten-4-olides作者:A. A. Avetisyan、A. N. Dzhandzhapanyan、R. G. Nazaryan、V. I. Votyakov、S. V. Khlyustov、G. V. Vladyko、V. Ya. Klimovich、L. V. Korobchenko、M. N. Shashikhina、S. V. ZhavridDOI:10.1007/bf00759749日期:1982.6spectrum of biological activity. Methods have been developed [12-16] for the preparation of substituted 2-buten-4-olides with a variety of function&l substituents. In order to obtain new compounds containing the 2-buten-4-olide ring and to examine their antiviral activity, we have carried out some chemical reactions of 2-acyl derivatives of 2-buten-4-olides (I-III), in whichthe lactone ring is conserved, as
-
Analgesic, anti-inflammatory, and antimicrobial activities of novel isoxazole/pyrimidine/pyrazole substituted benzimidazole analogs作者:Krishna Veni Chikkula、Raja SundararajanDOI:10.1007/s00044-017-2000-0日期:2017.11ligation method. The relationship between chemical structure and biological activities of the test compounds were discussed. Among various tested compounds, 4-(2-(4-(1H-benzimidazol-2-yl)phenyl)hydrazono)-1-(4-chlorophenyl)-3-methyl-1H-pyrazol-5(4H)-one 10 was found to be most potent compound. This compound showed 72% analgesic activity (20 mg/kg; second hour) and 67% protection in paw edema test (20 mg/kg;从邻苯二胺和对氨基苯甲酸中,几个新的3-甲基异恶唑-5(4 H)-一/ 2-羟基/巯基-6-甲基嘧啶-4(5 H)-一/ 3-甲基-1-取代-1 H-吡唑-5(4 H)-一取代的苯并咪唑衍生物5 – 16通过基于混合方法的多步合成来合成。分别通过甩尾法,角叉菜胶诱导的足爪浮肿法和琼脂条纹稀释法筛选所有测试化合物的镇痛,抗炎和体外抗菌活性。通过幽门结扎法检查了大多数活性化合物的致溃疡性。讨论了受试化合物的化学结构与生物学活性之间的关系。在各种测试的化合物中,4-(2-(4-(1 H-苯并咪唑-2-基)苯基)肼基)-1-(4-氯苯基)-3-甲基-1 H-吡唑-5(4 H)一个10被发现是最有效的化合物。该化合物显示出72%的镇痛活性(20 mg / kg;第二小时)和67%的爪水肿试验(20 mg / kg;第二小时)的保护作用,与标准双氯芬酸[69%的镇痛活性(20 mg / kg;第二小时
-
Aldimines of Cardenolides and Cardenolide-Glycosides作者:I. F. Makarevich、Yu. I. Gubin、R. MeggesDOI:10.1007/s10600-005-0142-7日期:2005.5New aldimines were synthesized from the cardenolide strophantidin and cardenolide-glycosides erysimin and cymarin and included morpholine, nitrile, pyridine, furan, hydroxy- and methoxyphenyl, piperidine, and other derivatives. An effective modified method for synthesizing aldimines was proposed. 52 new compounds were synthesized. Their structures were confirmed by IR and PMR spectra and elemental analysis.
-
Design, synthesis and anticancer evaluation of thieno[2,3-d]pyrimidine derivatives as dual EGFR/HER2 inhibitors and apoptosis inducers作者:Souad A. Elmetwally、Khaled F. Saied、Ibrahim H. Eissa、Eslam B. ElkaeedDOI:10.1016/j.bioorg.2019.102944日期:2019.7tyrosine kinase family is one of the most important targets in current cancer therapy regimens. In this study, we have designed and synthesized a series of thieno[2,3-d]pyrimidine derivatives as an EGFR and HER2 tyrosine kinase inhibitors. All the synthesized compounds were evaluated in vitro for their inhibitory activities against EGFRWT; and the most active compounds that showed promising IC50 values许多激酶的失调与癌症的发展直接相关,酪氨酸激酶家族是当前癌症治疗方案中最重要的靶标之一。在这项研究中,我们设计并合成了一系列噻吩并[2,3-d]嘧啶衍生物,作为EGFR和HER2酪氨酸激酶抑制剂。在体外评估所有合成的化合物对EGFRWT的抑制活性。并测试了对EGFRWT表现出有希望的IC50值的最具活性的化合物对突变EGFRT790M和HER2激酶的抑制活性。此外,测试了这些化合物对四种癌细胞系(HepG2,HCT-116,MCF-7和A431)的抗肿瘤活性。化合物13g,13h和13k对所检查的细胞系表现出最高活性,IC50值为7.592±0。相当于厄洛替尼的32至16.006±0.58 µM(IC50为4.99±0.09至13.914±0.36 µM)。此外,选择了最有效的抗肿瘤药(13k)进行进一步研究,以确定其对MCF-7细胞系中细胞周期进程和细胞凋亡的影响。结果表明,该化合物阻滞了细胞周期的G2
表征谱图
-
氢谱1HNMR
-
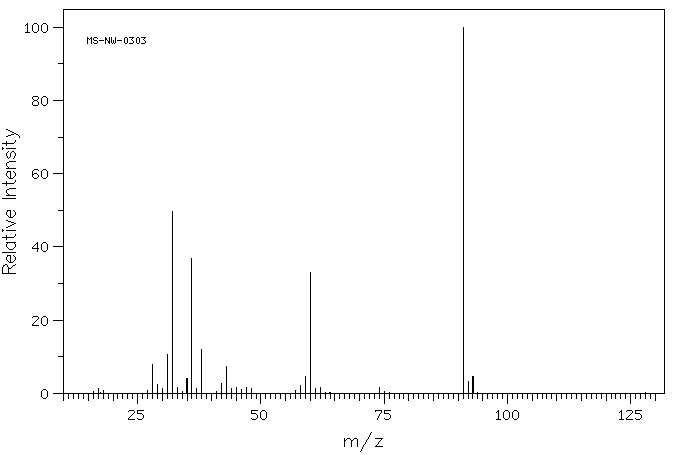
质谱MS
-
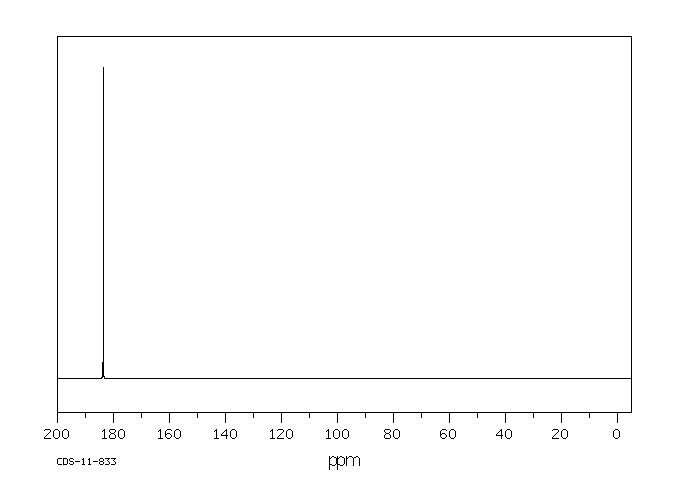
碳谱13CNMR
-
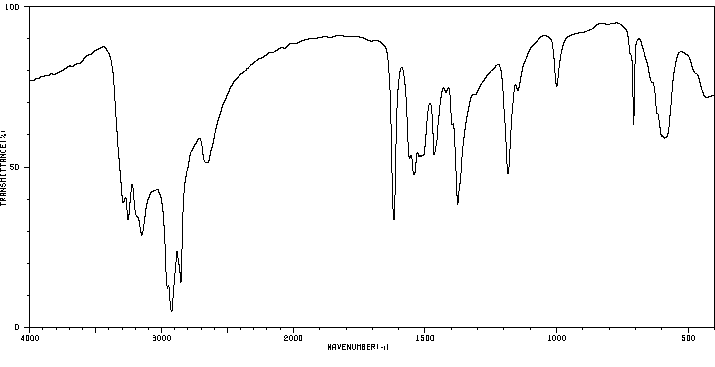
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷