1-苯基-乙酮 2-(2,4-二硝基苯基)腙 | 1677-87-8
中文名称
1-苯基-乙酮 2-(2,4-二硝基苯基)腙
中文别名
1-苯基-乙酮2-(2,4-二硝基苯基)腙
英文名称
acetophenone 2,4-dinitrophenylhydrazone
英文别名
1-(2,4-dinitrophenyl)-2-(1-phenylethylidene)-hydrazine;Acetophenone, (2,4-dinitrophenyl)hydrazone;2,4-dinitro-N-(1-phenylethylideneamino)aniline
CAS
1677-87-8
化学式
C14H12N4O4
mdl
MFCD00137171
分子量
300.274
InChiKey
IMTAQIPVTJOORO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:22
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:116
-
氢给体数:1
-
氢受体数:6
安全信息
-
海关编码:2928000090
SDS
上下游信息
反应信息
-
作为反应物:描述:1-苯基-乙酮 2-(2,4-二硝基苯基)腙 在 bismuth(III) chloride 、 benzyltriphenylphosphonium peroxymonosulfate 作用下, 以 乙腈 为溶剂, 反应 6.0h, 以40%的产率得到苯乙酮参考文献:名称:肟、苯腙、2,4-二硝基苯腙和亚氨基脲与苄基三苯基膦过氧单硫酸盐(BnPh3P+双锍-四氢呋喃)的相应羰基化合物的转化摘要:已发现苄基三苯基鏻过氧单硫酸盐 (BTPPMS) (1) 是一种有效的新型试剂,可用于将肟、苯腙、2,4-二硝基苯腙和缩氨基脲转化为相应的羰基化合物。该反应在乙腈中在回流条件下在催化量的氯化铋存在下进行。DOI:10.1081/scc-100106197
-
作为产物:描述:[(1-phenylethyl)thio]benzene 在 lithium aluminium tetrahydride 、 正丁基锂 、 间氯过氧苯甲酸 作用下, 以 四氢呋喃 、 正己烷 、 二氯甲烷 为溶剂, 反应 4.5h, 生成 1-苯基-乙酮 2-(2,4-二硝基苯基)腙参考文献:名称:通过α-甲硅烷基硫化物合成酮摘要:α-苯硫基硅烷(2)已经通过衍生自1-苯硫基-1-三甲基甲硅烷基烷(1)的阴离子(4)的烷基化而制备。这些阴离子(4)已经通过多种方法制备,包括:(1)的直接去质子化,萘锂置换苯硫基,在1-苯基硫-1-三甲基甲硅烷基乙烯中添加烷基锂(7),以及三丁基锡烷基部分的金属转移。通过萘二甲酸锂与苯硫醚的反应形成烷基锂,提供了从双(苯硫基)缩醛(8)到(2)的另一条路线。α-苯基硫代硅烷的替代途径(2)是还原相应的α-苯基磺酰基硅烷(15); 这些反过来很容易从α-磺酰基阴离子的烷基化或甲硅烷基化获得。通过sila-Pummerer重排将α-苯基硫代硅烷(2)转化为O-三甲基甲硅烷基苯基硫代缩醛(18),尽管在某些情况下这会因硫化乙烯基(20)的形成而变得复杂。随后将(18)和(20)水解,得到酮(3)。DOI:10.1039/p19860000195
文献信息
-
Barium Permanganate, Ba(MnO4)2, A Versatile and Mild Oxidizing Agent for Use Under Aprotic and Non-Aqueous Conditions作者:H. Firouzabadi、M. Seddighi、E. Mottaghineiad、M. BolourchianDOI:10.1016/s0040-4020(01)87874-1日期:1990.1versatile oxidation reagent. With this reagent different types of primary and secondary hydroxy compounds are converted to their carbonyl derivatives. Aldehydes could be transformed to their carboxylic acids. Benzylic chloride and bromides are converted to their aldehydes and carboxylic acids. Semicarbazide and 2,4-dinitrophenylhydrazine derivatives of benzylic carbonyl compounds undergo carbon-nitrogen
-
Microwave Assisted Facile Cleavage of 2,4-Dinitrophenylhydrazones to the Corresponding Carbonyl Compounds with N,N′-Dibromo-N,N′-1,2-ethanediylbis(p-toluenesulphonamide)作者:Ardeshir Khazaei、Ramin VagheiDOI:10.3390/70500465日期:——Deprotections of 2,4-dinitrophenylhydrazones to their corresponding carbonyl compounds have been carried out in good yields by using N,N′-dibromo-N,N′-1,2-ethanediylbis(p-toluenesulphonamide) (BNBTS, 2) under microwave irradiation.
-
Facile preparation and reactivity of polystyrene-supported (dichloroiodo)benzene: a convenient recyclable reagent for chlorination and oxidation作者:Jiang-Min Chen、Xiao-Mei Zeng、Kyle Middleton、Viktor V. ZhdankinDOI:10.1016/j.tetlet.2011.02.065日期:2011.4of polystyrene-supported (dichloroiodo)benzene (loading of -ICl2 up to 1.35 mmol/g) from polystyrene, iodine, and bleach has been developed. This recyclable reagent is useful for efficient chlorination of organic substrates and selective oxidation of various alcohols to the corresponding carbonyl compounds in high yields under mild conditions. The final products are conveniently separated from the polymeric
-
2-Iodoxybenzoic acid organosulfonates: preparation, X-ray structure and reactivity of new, powerful hypervalent iodine(v) oxidants作者:Mekhman S. Yusubov、Dmitrii Yu. Svitich、Akira Yoshimura、Victor N. Nemykin、Viktor V. ZhdankinDOI:10.1039/c3cc47090c日期:——New powerful hypervalent iodine(V) oxidants, tosylate and mesylate derivatives of 2-iodoxybenzoic acid (IBX), were prepared by the reaction of IBX with the corresponding sulfonic acids. Single crystal X-ray crystallography of the diacetate derivative of IBX-tosylate revealed an unusual heptacoordinated iodine geometry without any significant intermolecular secondary interactions.
-
Synthesis and properties of 4,9-methanoundecafulvenes and their transformation to 3-substituted 7,12-methanocycloundeca[4,5]furo[2,3-d]pyrimidine-2,4(1H,3H)-diones: photo-induced autorecycling oxidizing reaction toward amines作者:Shin-ichi Naya、Yohei Yamaguchi、Makoto NittaDOI:10.1016/j.tet.2005.05.069日期:2005.8NMR measurement. The electrochemical properties of 8a,b were also studied by CV measurement. Furthermore, the transformation of 8a,b to 3-substituted 7,12-methanocycloundeca[4,5]furo[2,3-d]pyrimidine-2,4(1H,3H)-diones 16a,b was accomplished by oxidative cyclization using DDQ and subsequent ring-opening and ring-closure. The structural details and chemical properties of 16a,b were clarified. Reaction4,9-甲基呋喃二烯[5-(4,9-甲基环己烯-2',4',6',8',10'-戊烯叉基)嘧啶-2,4,6(1,3,5研究了H)-三酮]衍生物8a,b。根据1 H和13 C NMR和UV-vis光谱研究了它们的结构特征。通过可变温度1 H NMR测量发现,围绕8a的环外双键的旋转势垒(ΔG ‡)为12.55kcal mol -1。还通过CV测量研究了8a,b的电化学性质。此外,改造图8a,b,以3-取代的7,12- methanocycloundeca [4,5]呋喃并[2,3- d ]嘧啶-2,4(1 ħ,3 ħ) -二酮16a中,b是通过使用DDQ和氧化环化来完成随后的开环和闭环。阐明了16a,b的结构细节和化学性质。16a与氘化物的反应提供了C13加合物19作为单一产物,因此,甲醇桥控制亲核攻击,以偏爱内在选择性。16a与乙烯基化合物3-甲基环庚[4,5]呋喃[2,3-]的光诱导氧化反应d
表征谱图
-
氢谱1HNMR
-
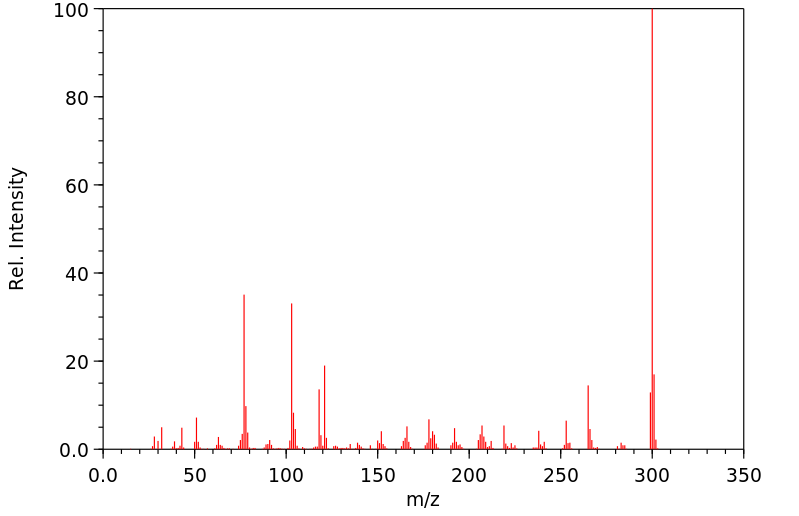
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫