11-甲基苯[a]蒽 | 6111-78-0
中文名称
11-甲基苯[a]蒽
中文别名
——
英文名称
11-methylbenz[a]anthracene
英文别名
CAS
6111-78-0
化学式
C19H14
mdl
——
分子量
242.32
InChiKey
ARHCZXQENFEAFA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:118°C
-
沸点:452.14°C (rough estimate)
-
密度:1.1011 (estimate)
-
保留指数:412.88;412.05;412.72;412.72;412.72
计算性质
-
辛醇/水分配系数(LogP):6.3
-
重原子数:19
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.05
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902909090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:THE SYNTHESIS OF 7,9,10-TRIMETHYL-1,2-BENZANTHRACENE AND 8,9,10-TRIMETHYL-1,2-BENZANTHRACENE1摘要:DOI:10.1021/jo01201a003
-
作为产物:描述:参考文献:名称:Synthesis of 1′,9-Methylene-1,2-benzanthracene and Related Hydrocarbons摘要:DOI:10.1021/ja01876a031
文献信息
-
Solvolysis of K-region arene oxides: substituent effects on reactions of benz[a]anthracene 5,6-oxide作者:Nashaat T. Nashed、Suresh K. Balani、Richard J. Loncharich、Jane M. Sayer、David Y. Shipley、Ram S. Mohan、Dale L. Whalen、Donald M. JerinaDOI:10.1021/ja00010a036日期:1991.5dioxane-water and in methanol at 25 o C are reported. These substitutions result in >150-fold differences in their rates of acid-catalyzed solvolysis and cause marked changes in the distribution of solvent adducts and phenols resulting from isomerization. Optically pure BA-O, 7-MBA-O, 12-MBA-O, and 7,12-DMBA-O as well as their optically pure trans dihydrodiols were utilized to determine the point of attack by
-
Substituent Effects in Benz[<i>a</i>]anthracene Carbocations: A Stable Ion, Electrophilic Substitution (Nitration, Bromination), and DFT Study作者:Kenneth K. Laali、Maria A. Arrica、Takao Okazaki、Ronald G. HarveyDOI:10.1021/jo070936r日期:2007.8.31computed relative energies by DFT. Charge delocalization paths in the resulting carbocations were deduced based on the magnitude of Δδ13C values. For the thermodynamically more stable C-12 protonated carbocations, the charge delocalization path is analogous to those derived based on computed NPA charges for the benzylic carbocations formed by 1,2-epoxide (bay-region) and 5,6-epoxide (K-region) ring opening在FSO 3 H / SO 2 ClF中通过低温质子化作用,由异构的单烷基化和二烷基化的苯并[ a ]蒽(BAs)生成了一系列新型的碳正离子化反应。C-7具有单烷基衍生物(5-甲基,6-甲基,7-甲基和7-乙基)以及D环甲基化类似物(9-甲基,10-甲基和11-甲基),或在所有情况下均观察到C-12质子化的碳正离子(作为唯一或主要的碳正离子)。12-甲基衍生物的质子化(9)得到C-7质子化的碳正离子(9H +)作为动能种类和本位-protonated碳阳离子(9AH +)作为热力学阳离子。与12-乙基衍生物(10),在箱式区域空间张力的浮雕大大有利于本位-protonation(10AH +)。具有3,9-二甲基(14),C-7质子化(14H +)(C-12质子化<10%)受到强烈青睐,在1,12-二甲基(15)的情况下,观察到的唯一物质是C-7质子化的碳正离子化(15H +)。对于7-甲
-
Cascade reaction for the synthesis of polycyclic aromatic hydrocarbons via transient directing group strategy作者:Ziqi Wang、Wendan Dong、Bing Sun、Qinqin Yu、Fang-Lin ZhangDOI:10.1016/j.tet.2019.06.036日期:2019.7A Pd(II)-catalyzed cascade synthesis of diverse polycyclic aromatic hydrocarbons via transient directing group strategy has been developed, involving the consecutive arylation, cyclization and aromatization. The efficiency and practicality were demonstrated by wide substrate range, concise synthetic pathway and mild reaction conditions. The subsequent transformations of the benz[a]anthracene core accessed
-
105. Polycyclic aromatic hydrocarbons. Part XVII. Completion of the synthesis of the twelve monomethyl-1 : 2-benzanthracenes作者:J. W. Cook、A. M. RobinsonDOI:10.1039/jr9380000505日期:——
-
Coördination of Polycyclic Aromatic Hydrocarbons with Silver Ion; Correlation of Equilibrium Constants with Relative Carcinogenic Potencies<sup>1</sup>作者:Robert E. Kofahl、Howard J. LucasDOI:10.1021/ja01644a020日期:1954.8
表征谱图
-
氢谱1HNMR
-
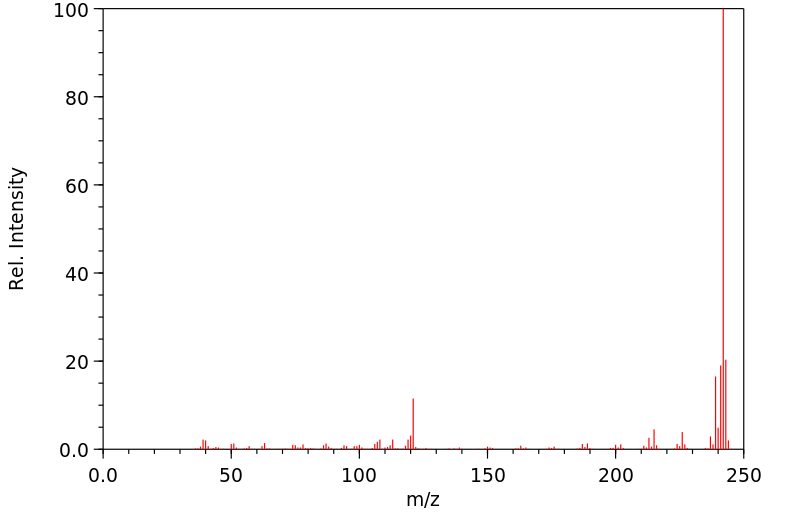
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(6,6)-苯基-C61己酸甲酯
高雌二醇
马兜铃酸钠
马兜铃酸盐
马兜铃酸C
马兜铃酸B
马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII)
马兜铃酸 Ia
马兜铃酸 IVa
马兜铃酸
颜料黑32
颜料红179
颜料红178
颜料红149
颜料红123
顺式-菲-1,2-二醇-3,4-环氧化物
顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物
雷公藤酚A
镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯)
钩大青酮
钩大青酮
钙(2+)12-羟基十八烷酸酯
酒石酸布托诺啡
那布扶林
还原红32
足球烯
贝那他汀B
贝母兰素
萘并[2,3-b]荧蒽
萘并[2,1-e][1]苯并二硫杂环戊烷
萘并[2,1-C:7,8-C']二菲
萘并[1,2-e][2]苯并呋喃-1,3-二酮
萘并[1,2-b]屈
萘并[1,2-a]蒽
萘并[1,2-B]菲-6-醇
萘二(六氯环戊二烯)加合物
萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基-
菲醌单缩氨基硫脲
菲醌
菲并[9,10]呋喃
菲并[9,10-e]醋菲烯
菲并[4,5-bcd]噻吩
菲并[4,5-bcd]呋喃-3-醇
菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基-
菲并[3,2-b]噻吩
菲并[2,1-d]噻唑
菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮
菲并(3,4-b)噻吩
菲并(1,2-b)噻吩