氯乙腈 | 107-14-2
中文名称
氯乙腈
中文别名
氰化氯甲烷;氯甲基氰;氯乙酰腈;一氯乙腈
英文名称
chloroacetonitrile
英文别名
Chloracetonitrile;2-chloroacetonitrile;choloroacetonitrile
CAS
107-14-2
化学式
C2H2ClN
mdl
MFCD00001885
分子量
75.4976
InChiKey
RENMDAKOXSCIGH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:38℃
-
沸点:124-126 °C (lit.)
-
密度:1.193 g/mL at 25 °C (lit.)
-
蒸气密度:3 (vs air)
-
闪点:118 °F
-
溶解度:可溶于氯仿、乙酸乙酯
-
介电常数:30.0
-
暴露限值:NIOSH: IDLH 14 ppm(25 mg/m3)
-
LogP:0.45
-
物理描述:Chloroacetonitrile appears as a colorless liquid with a pungent odor. Flash point 118°F. Insoluble in water and denser than water. Hence, sinks in water. Very toxic by ingestion, inhalation and skin absorption. A lachrymator. Used to make other chemicals and as a fumigant.
-
颜色/状态:Colorless liquid
-
气味:Pungent odor
-
蒸汽密度:2.61 (Air = 1)
-
蒸汽压力:8 mm Hg at 20 °C
-
分解:When heated to decomposition it emits very toxic fumes of /chlorides, nitrogen oxides, and cyanides/.
-
折光率:Index of refraction: 1.4210-1.4240 at 25 °C.
-
保留指数:635.5;661.7
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:23.8
-
氢给体数:0
-
氢受体数:1
ADMET
代谢
Chloroacetonitirile is metabolized to hydrogen cyanide by mouse hepatic microsomal fractions.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Approximately 14% of a single oral dose to rats of 57 mg/kg body weight of chloroacetonitrile was excreted in urine within 24 hours as thiocyanate, the product of released cyanide metabolized by rhodanese.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Haloacetonitriles, contaminants present in chlorinated drinking water, were administered orally to rats, and the urinary excretion of thiocyanate was measured as an index of cyanide release. The urinary excretion of thiocyanate accounted for 14.2% of the dose of monochloroacetonitrile ...
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Evaluation: No epidemiological data relevant to the carcinogenicity of chloroacetonitrile were available. There is inadequate evidence for the carcinogenicity of chloroacetonitrile in experimental animals. Overall evaluation: Chloroacetonitrile is not classifiable as to its carcinogenicity to humans (Group 3).
来源:Hazardous Substances Data Bank (HSDB)
毒理性
国际癌症研究机构致癌物:氯乙腈
IARC Carcinogenic Agent:Chloroacetonitrile
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:第3组:无法归类其对人类致癌性
IARC Carcinogenic Classes:Group 3: Not classifiable as to its carcinogenicity to humans
来源:International Agency for Research on Cancer (IARC)
毒理性
IARC Monographs:Volume 52: (1991) Chlorinated Drinking-water; Chlorination By-products; Some Other Halogenated Compounds; Cobalt and Cobalt Compounds
来源:International Agency for Research on Cancer (IARC)
毒理性
该物质可以通过吸入其气溶胶、通过皮肤和经口摄入被身体吸收。
The substance can be absorbed into the body by inhalation of its aerosol, through the skin and by ingestion.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
吸收、分配和排泄
... 雄性Sprague-Dawley大鼠以示踪剂量的2-(14)C-氯乙酰腈(CAN) (静脉注射, 88微居里/千克, 比活度4.07毫居里/毫摩尔)进行处理。在处理后不同的时间间隔(0.08、1、3、6、12、24和48小时), 对大鼠进行整体放射自显影(WBA)处理。给药12小时后, 尿液中排出的放射性占剂量的51%, 粪便中占2.7%, 作为(14)CO2呼出的占12%。仅有0.8%的给药剂量以未改变的CAN形式呼出。在早期时间间隔(5分钟)观察到肝脏、肾脏和胃肠(G.I.)壁中放射性物质的广泛积累。此外, 甲状腺、肺细支气管、肾上腺皮质、唾液腺和睾丸中检测到高水平的(14)C。给药后1小时, 嗅球、大脑的嗅觉受体区域和腰椎池显示出放射性CAN或其等效物的 高积累。在处理后3、6和12小时, 放射性物质在所有组织中均匀扩散, 并在较晚的时间段(24和48小时)在几个器官中重新浓缩。... 甲状腺、G.I.、睾丸、大脑和眼睛组织中放射性物质的保留表明, 这些器官可能是CAN毒性的潜在靶点。
... Male Sprague-Dawley rats were treated with a tracer dose of 2-(14)C-/chloroacetonitirile (CAN)/ (i.v., 88 muCi/kg, spec. act 4.07 mCi/mM). At various time intervals (0.08, 1, 3, 6, 12, 24, and 48 hr) after treatment, rats were processed for WBA, /whole body autoradiography/. Over 12 hr after administration, the radioactivity excreted in urine, feces, and exhaled as (14)CO2 accounted for 51%, 2.7%, and 12% of the dose, respectively. Only 0.8% of the administered dose was exhaled as unchanged CAN. At an early time interval (5 min) extensive accumulation of radioactivity was observed in liver, kidney, and gastrointestinal (G.I.) walls. In addition, high levels of (14)C were detected in the thyroid gland, lung bronchioles, adrenal cortex, salivary gland, and testes. At 1 hr following administration, the olfactory bulb, olfactory receptor area of the brain and lumbar cistern showed high accumulations of radioactive CAN or its equivalents. At 3, 6, and 12 hr after treatment, the radioactivity diffused homogeneously in all tissues and reconcentrated in several organs at later time periods (24 and 48 hr). ... The retention of radioactivity in the tissues of the thyroid gland, G.I., testes, brain and eye suggest that those organs are potential target sites of CAN toxicity.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
调查人员试图通过检查正常和谷胱甘肽(GSH)耗竭的怀孕小鼠(妊娠第13天)中化学物质的处置、跨胎盘摄取和共价相互作用来理解这种分子相互作用中涉及的潜在机制。正常和GSH耗竭(通过给予二乙基马来酸酯(DEM),0.6 mL/kg,i.p.)的怀孕小鼠被给予等毒性的静脉注射剂量的2-(14)C-CAN(333 uCi/kg相当于77 mg/kg)。动物在处理后1、8和24小时进行全身自动放射性显影(WBA)。使用计算机辅助图像分析对自动放射性显影中的放射性活性的组织分布进行量化。除少数例外,在1小时内在所有主要母体(肝脏、肺、膀胱、胃肠粘膜、小脑、子宫腔液)和胎儿(肝脏、大脑)器官中观察到放射性活性的快速高摄取。这种分布模式在治疗后8小时以较低强度观察到。在较晚的时间段(24小时),GSH耗竭的小鼠组织中放射性活性的显著更高保留和共价相互作用,特别是在肝脏与正常小鼠相比。这项研究表明,2-(14)C-CAN及其代谢物能够跨越胎盘屏障。观察到的在母体肝脏、肾脏、小脑、鼻甲和胎儿肝脏中的放射性活性的更高摄取和保留可能对化学物质对这些器官的毒性构成风险。GSH耗竭的小鼠肝脏中放射性活性的增加共价相互作用可能表明该器官可能利用GSH途径来解毒CAN衍生代谢物,从而产生肝毒性。
... /Investigators sought to/ understand the potential mechanisms involved in such molecular interactions by examining the disposition, transplacental uptake and covalent interaction of the chemical in normal and GSH depleted pregnant mice (at 13th day of gestation). Both normal and GSH depleted (by administration of Diethylmaleate (DEM), 0.6 mL/kg, i.p.) pregnant mice were given an equitoxic i.v. dose of 2-(14)C-CAN (333 uCi/kg equivalent to 77 mg/kg). Animals were processed for whole-body autoradiography (WBA) at 1, 8 and 24 hr after treatment. Tissue distribution of radioactivity in the autoradiographs was quantitated using computer aided image analysis. With few exceptions, a rapid high uptake (at 1 hr) of radioactivity was observed in all major maternal (liver, lung, urinary bladder, gastrointestinal mucosa, cerebellum, uterine luminal fluid) and fetal (liver, brain) organs of both normal and GSH depleted mice. This pattern of distribution was observed, with lesser intensity, at 8 hr following treatment. At a later time period (24 hr), there was a significant higher retention and covalent interaction of radioactivity in GSH depleted mouse tissues especially in the liver as compared to normal mouse. This study suggests that 2-(14)C-CAN and/or its metabolites are capable of crossing the placental barrier. The observed higher uptake and retention of the radioactivity in the maternal liver, kidney, cerebellum, nasal turbinates and fetal liver may pose toxicity of the chemical to these organs. The increased covalent interaction of radioactivty in GSH depleted mice liver may indicate the potential utilization of GSH pathway by this organ in the detoxication of CAN derived metabolites and thus exerting hepatotoxicity.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
立即威胁生命和健康浓度:14 ppm
-
危险品标志:T
-
安全说明:S45,S61
-
危险类别码:R23/24/25,R51/53
-
WGK Germany:3
-
海关编码:2926909090
-
危险品运输编号:UN 2668 6.1/PG 2
-
危险类别:6.1
-
RTECS号:AL8225000
-
包装等级:II
-
储存条件:储存于阴凉、干燥且通风良好的库房中。远离火种和热源,保持容器密封。与氧化剂、还原剂、酸类、碱类及食用化学品分开存放,避免混合储藏。使用防爆型照明和通风设施,并禁止使用可能产生火花的机械设备和工具。储存区应配备泄漏应急处理设备和合适的收容材料。
制备方法与用途
氯乙腈
氯乙腈亦称“氰化氯甲烷”,化学式为ClCH2CN,分子量75.50,是一种无色发烟液体。其熔点为38℃,沸点在126~127℃(分解)或30~32℃(2.0kPa),相对密度1.1930,折光率1.420225。它溶于醚、醇及烃类,而不溶于水。氯乙腈具有极高的毒性,并能与三氯化铝形成加合物,还可以与均苯三酚三甲醚、甲氧基苯乙酮、格氏试剂以及无水氯化氢等多种试剂反应。
氯乙腈可通过将氯乙酰胺在五氧化二磷的作用下脱水制得,或由过量的乙腈在460℃与氯气反应制备。它还能够通过将氨气导入二氯乙炔的乙醚溶液中获得。氯乙腈可作为熏蒸剂使用。
熏蒸剂熏蒸剂是指利用挥发时所产生的蒸气毒杀有害生物的一类农药,其作用机制在于使害虫体内的酶发生化学抑制从而导致死亡。常见的熏蒸剂包括溴甲烷、磷化氢等。熏蒸剂量通常根据熏蒸场所的空间体积计算(单位为克/米³),而浓度则需考虑熏蒸时间、场所密闭程度以及被熏蒸物品的量和对熏蒸剂吸附的能力等因素。
化学性质氯乙腈是一种无色透明发烟液体,具有刺激性呛味,并可溶于醇和乙醚。
用途氯乙腈主要用于有机合成原料及分析试剂。此外,它还用作医药中间体、杀虫剂以及有机合成的中间体,在某些情况下也用于有机合成并作为分析试剂使用。
生产方法生产氯乙腈的方法如下:首先由氯乙酸与乙醇反应生成氯乙酸乙酯,然后在0-2℃的氨水中继续反应生成氯乙酰胺。随后将氯乙酰胺与五氧化二磷一同加热脱水,在不断蒸出氯乙腈的过程中进行减压蒸馏以使其全部蒸发。最后经过干燥处理并进一步分馏以获得成品。
类别 毒性分级- 毒性:高毒
-
急性毒性:
- 大鼠口服 LD50: 220毫克/公斤;
- 小鼠口服 LD50: 139毫克/公斤
- 皮肤刺激(兔子):14毫克/24小时 轻度
- 眼部刺激(兔子):20毫克/24小时 中度
- 库房应通风低温干燥;
- 需与氧化剂、酸类及食品添加剂分开存放。
- 干粉、干石粉、二氧化碳、砂土。禁用酸碱灭火剂。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 溴代氯乙腈 bromochloroacetonitrile 83463-62-1 C2HBrClN 154.394
反应信息
-
作为反应物:参考文献:名称:一种氨基乙腈盐酸盐的制备方法摘要:本发明公开了一种氨基乙腈盐酸盐的制备方法,包括以下步骤:①将氯乙腈与氨反应得到氨基乙腈;②将步骤①得到的氨基乙腈与氯化氢、盐酸、盐酸甲醇或盐酸乙醇中的一种反应生成氨基乙腈盐酸盐。本发明设计的氨基乙腈盐酸盐的合成路线,以氯乙腈为原料,与氨反应生成氨基乙腈,再和酸反应生成氨基乙腈盐酸盐;反应原料不使用剧毒的氢氰酸或氰化钠,避免氢氰酸或氰化钠路线废水难处理的问题。公开号:CN117164479A
-
作为产物:参考文献:名称:一种合成氯氰甲烷的新方法摘要:本发明公开了一种合成氯氰甲烷的新方法,属于合成技术领域。该方法步骤为:首先将具有通式(Ⅱ)的化合物原料溶于溶剂中,然后加入氯化试剂进行氯化反应,反应结束后反应液不经处理;然后向水中加入催化剂,将氯化反应结束后的反应液滴加至水中,进行水解脱羧反应,反应结束后经提纯即可得氯氰甲烷。本发明方法反应条件温和,产品收率高,产品质量好。公开号:CN111393325A
-
作为试剂:参考文献:名称:可结合到链霉亲和素特定位置的高灵敏度荧光非天然氨基酸摘要:合成了一种带有邻氨基苯甲酰基的小且高度荧光的非天然氨基酸,并将其掺入链霉亲和素的特定位置。荧光氨基酸的位置由CGGG/CCCG四碱基密码子/反密码子对指示。含有四碱基反密码子并带有荧光氨基酸的 tRNA 制备如图 1 所示。简而言之,关键中间体(pdCpA 连接的氨基酸)3 被合成并与 tRNACCCG(-CA) 偶联T4 RNA 连接酶。然后,将氨酰化的 tRNA 4 与大肠杆菌 S30 裂解物的体外生物合成系统以及含有四碱基的突变链霉亲和素 mRNA 混合。DOI:10.1080/10426500213445
文献信息
-
腈及其相应胺的制造方法申请人:中国石油化工股份有限公司公开号:CN104557610B公开(公告)日:2018-04-27本发明涉及一种腈的制造方法,与现有技术相比,具有氨源用量显著降低、环境压力小、能耗低、生产成本低、腈产物的纯度和收率高等特点,并且能够获得结构更为复杂的腈。本发明还涉及由该腈制造相应胺的方法。
-
Sulphur- and oxygen-containing diaryl compounds申请人:Societe Anonyme dite: Laboratoire L. Lafon公开号:US04156011A1公开(公告)日:1979-05-22The sulphur- and oxygen-containing diaryl compounds of the formula: ##STR1## in which A and B, which may be the same or different, represent O, S, SO or SO.sub.2, Alk is a C.sub.1 -C.sub.4 hydrocarbon radical with a straight or branched chain, R represents COOH, an esterified COOH group, a carboxylic amide group, OH, O-SO.sub.2 CH.sub.3, NH.sub.2, NHR.sub.1, NR.sub.1 R.sub.2, NHZOH, NHZNR.sub.1 R.sub.2, C(.dbd.NH)NH.sub.2, C(.dbd.NH)NHOH or 2-.DELTA..sup.2 -imidazolinyl, Z is a C.sub.2 -C.sub.4 hydrocarbon radical with a straight or branched chain, and R.sub.1 and R.sub.2 each represent a C.sub.1 -C.sub.3 lower alkyl group, or together form, with the nitrogen atom to which they are linked, a N-heterocyclic group of 5 to 7 ring atoms which can be substituted and can comprise a second hetero-atom, and their addition salts with bases when R is COOH, and their addition salts with acids when R is a basic radical, are useful pharmacological agents in the treatment of circulatory complaints such as cardio-vascular illnesses.公式为:##STR1## 其中A和B(可以相同也可以不同)代表O、S、SO或SO.sub.2,Alk是一种直链或支链的C.sub.1-C.sub.4烃基,R代表COOH、酯化的COOH基团、羧酰胺基团、OH、O-SO.sub.2 CH.sub.3、NH.sub.2、NHR.sub.1、NR.sub.1 R.sub.2、NHZOH、NHZNR.sub.1 R.sub.2、C(.dbd.NH)NH.sub.2、C(.dbd.NH)NHOH或2-.DELTA..sup.2-咪唑啉基,Z是一种直链或支链的C.sub.2-C.sub.4烃基,R.sub.1和R.sub.2各自代表C.sub.1-C.sub.3较低的烷基基团,或者与它们连接的氮原子一起形成由5到7个环原子组成的N-杂环基团,该基团可以被取代并且可以包含第二个杂原子,当R为COOH时,它们与碱形成的加合盐,当R为碱性基团时,它们与酸形成的加合盐,在治疗心血管疾病等循环系统疾病方面是有用的药理剂。
-
[EN] METHODS OF TREATMENT OF AMYLOIDOSIS USING ASPARTYL-PROTEASE INIHIBITORS<br/>[FR] PROCEDES DE TRAITEMENT D'AMYLOIDOSE UTILISANT DES INHIBITEURS DE PROTEASE ASPARTYLE申请人:ELAN PHARM INC公开号:WO2005070407A1公开(公告)日:2005-08-04The invention relates to acetyl 2-hydroxy-1,3-diaminospirocyclohexanes and derivatives thereof that are useful in treating diseases, disorders, and conditions associated with amyloidosis. Amyloidosis refers to a collection of diseases, disorders, and conditions associated with abnormal deposition of A-beta protein.
-
Design, synthesis, and biological activity studies of a new class of sulfonated aurones: First synthesis of acidoaurone isolated from<scp><i>Phyllanthus acidus</i></scp>作者:Somepalli Venkateswarlu、Gandrotu Narasimha Murty、Meka Satyanarayana、Vidavalur SiddaiahDOI:10.1002/jhet.4358日期:2021.12comparison with their corresponding natural aurones. Ring-B sulfonated aurones exhibited potent 5-LOX inhibitory activity and significant antioxidant activity. Acidoaurone, a first sulfonated aurone isolated from Phyllanthus acidus was synthesized for the first time and was well characterized using NMR, LC–MS, and further confirmed by HMBC.通过在已知的天然aurones,如hispidol、suretin、seastin和aureusidin的环-A和环-B上引入磺酸基团,设计了一系列新的aurones。这些磺化的金黄酮是通过一种独特的方法合成的。环-A 或环-B 上的磺化作用将不溶于水的金黄酮转化为高度溶于水的金黄酮。测试了磺化的 aurone 及其天然 aurone 的抗氧化、抗炎和 AChE 抑制活性。与相应的天然金酮相比,环 A 磺化的金酮显示出更高的抗氧化活性、5-LOX 和 AChE 抑制。环 B 磺化的 aurone 表现出有效的 5-LOX 抑制活性和显着的抗氧化活性。Acidoaurone,第一个从Phyllanthus acidus 中分离出来的磺化的 aurone 首次合成并使用 NMR、LC-MS 进行了充分表征,并通过 HMBC 进一步确认。
-
PHARMACEUTICAL COMPOUNDS AS INHIBITORS OF CELL PROLIFERATION AND THE USE THEREOF申请人:ANDERSON MARK B.公开号:US20100068197A1公开(公告)日:2010-03-18Disclosed are compounds of Formula I effective as cytotoxic agents. The compounds of this invention are useful in the treatment of a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.揭示的是作为细胞毒性剂有效的I式化合物。本发明的化合物在治疗多种临床病况中是有用的,这些病况中发生异常细胞的不受控制的生长和扩散。
表征谱图
-
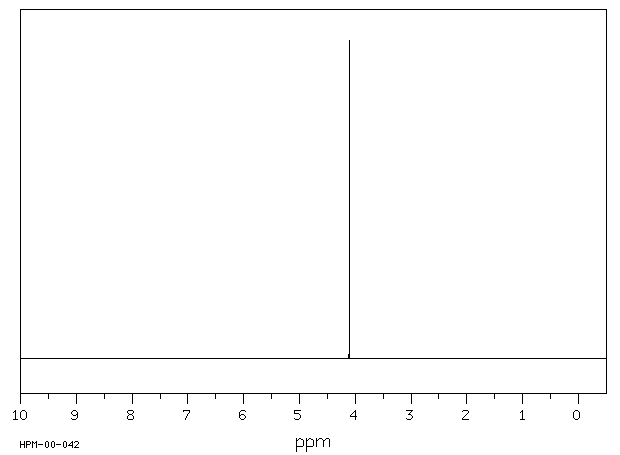
氢谱1HNMR
-
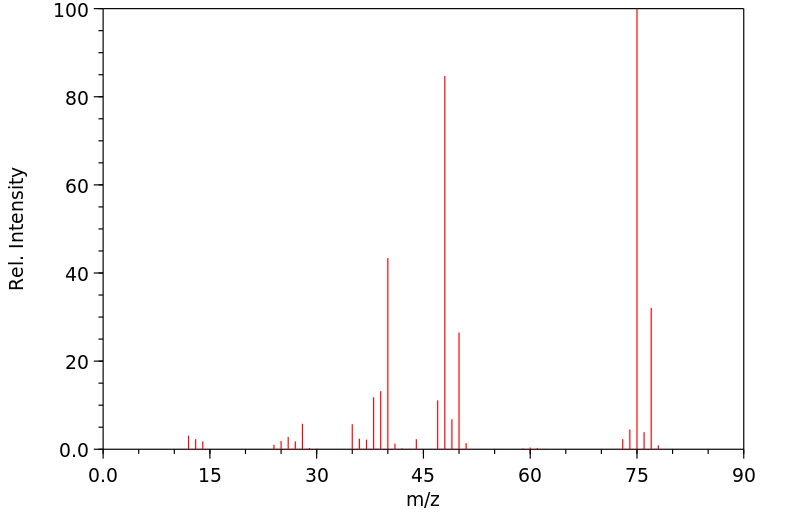
质谱MS
-
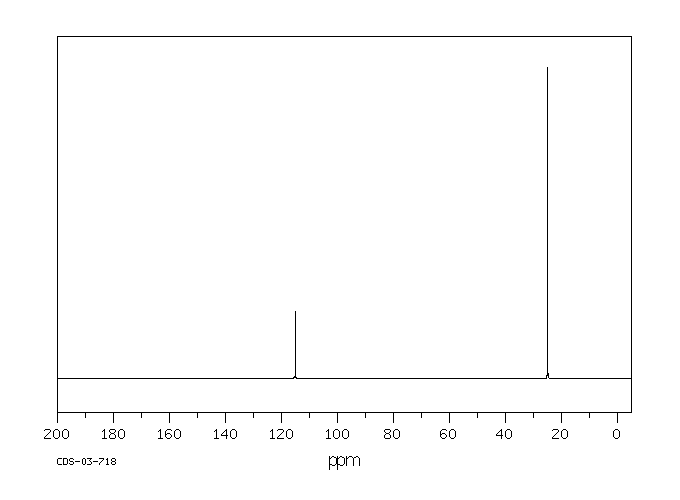
碳谱13CNMR
-
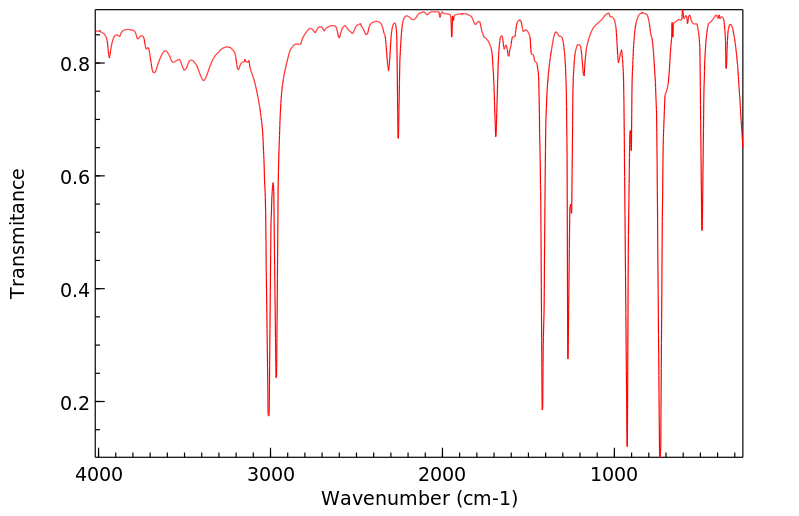
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷