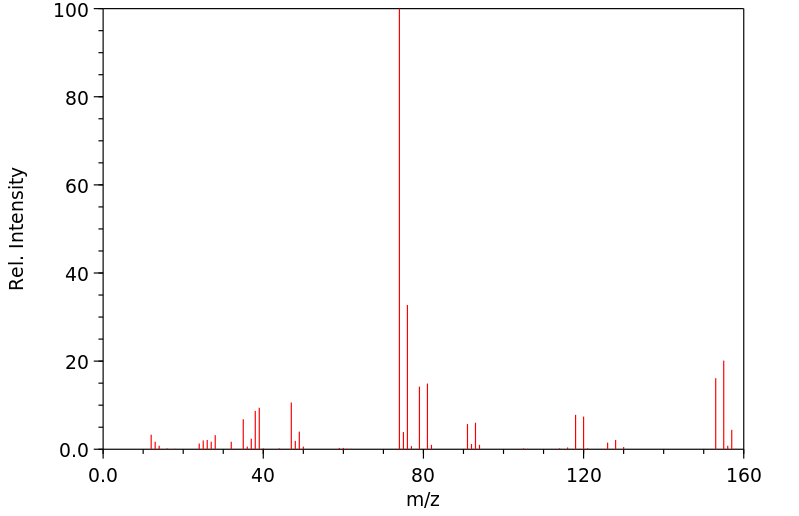
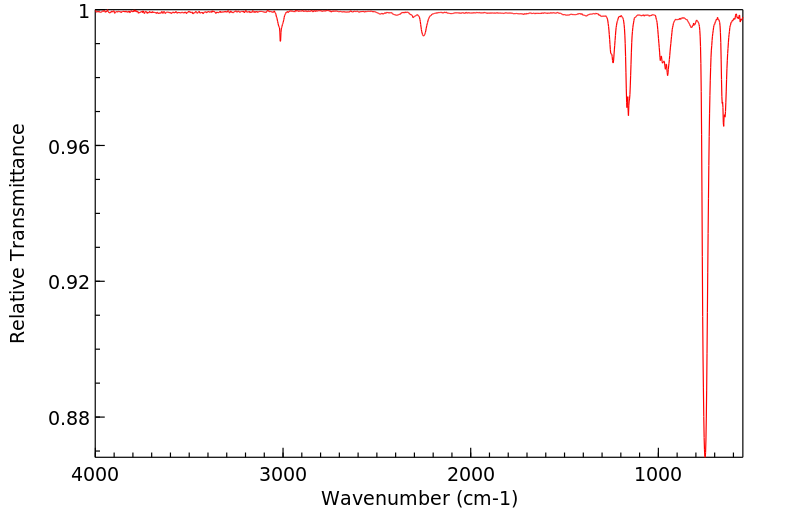
代谢
当大鼠口服卤乙腈(HAN)时,它们被代谢成氰化物,并作为硫氰酸盐通过尿液排出。硫氰酸盐排出的程度是氯乙腈(CAN)大于溴氯乙腈(BCAN)大于二氯乙腈(DCAN)大于二溴乙腈(DBAN)远大于三氯乙腈(TCAN)。
When administered orally to rats, the haloacetonitriles (HAN) were metabolized to cyanide and excreted in the urine as thiocyanate. The extent of thiocyanate excretion was chloroacetonitrile (CAN) greater than bromochloroacetonitrile (BCAN) greater than dichloroacetonitrile (DCAN) greater than dibromoacetonitrile (DBAN) much greater than trichloroacetonitrile (TCAN).
来源:Hazardous Substances Data Bank (HSDB)