2,3-二(羟甲基)萘 | 31554-15-1
中文名称
2,3-二(羟甲基)萘
中文别名
2,3-萘二甲醇;2,3-双(羟甲基)萘
英文名称
2,3-bis(hydroxymethyl)naphthalene
英文别名
2,3-Bis(hydroxymethyl)naphthalin;2,3-naphthalene dimethanol;naphthalene-2,3-diyldimethanol;[3-(hydroxymethyl)naphthalen-2-yl]methanol
CAS
31554-15-1
化学式
C12H12O2
mdl
MFCD00191314
分子量
188.226
InChiKey
DTYJRRQQOLBRGH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:157 °C
-
沸点:400.9±25.0 °C(Predicted)
-
密度:1.244±0.06 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,也未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:14
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2906299090
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
2,3-双(羟甲基)萘 修改号码:5
模块 1. 化学品
产品名称: 2,3-Bis(hydroxymethyl)naphthalene
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2,3-双(羟甲基)萘
百分比: >96.0%(GC)
CAS编码: 31554-15-1
分子式: C12H12O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
2,3-双(羟甲基)萘 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-浅黄色
气味: 无资料
pH: 无数据资料
熔点:
157°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
2,3-双(羟甲基)萘 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
2,3-双(羟甲基)萘 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2,3-Bis(hydroxymethyl)naphthalene
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2,3-双(羟甲基)萘
百分比: >96.0%(GC)
CAS编码: 31554-15-1
分子式: C12H12O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
2,3-双(羟甲基)萘 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-浅黄色
气味: 无资料
pH: 无数据资料
熔点:
157°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
2,3-双(羟甲基)萘 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
2,3-双(羟甲基)萘 修改号码:5
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,3-萘二羧酸 naphthalene-2,3-dicarboxylic acid 2169-87-1 C12H8O4 216.193 2,3-萘基二缩醛 2,3-naphthalenedicarboxaldehyde 7149-49-7 C12H8O2 184.194 2,3-萘磺酸二甲基乙酸酯 dimethyl 2,3-naphthalenedicarboxylate 13728-34-2 C14H12O4 244.247 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (3-羟甲基蒽-2-基)-甲醇 2,3-bis(hydroxymethyl)anthracene 1134642-63-9 C16H14O2 238.286 —— 1,3-dihydro{naphtho-[2,3-c]}furan 7193-16-0 C12H10O 170.211 2,3-萘基二缩醛 2,3-naphthalenedicarboxaldehyde 7149-49-7 C12H8O2 184.194 2,3-双(氯甲基)萘 2,3-bis(chloromethyl)naphthalene 2744-60-7 C12H10Cl2 225.117 萘并[2,3-c]呋喃-3(1H)-酮 naphtho[2,3-c]furan-1(3H)-one 4711-50-6 C12H8O2 184.194 蒽-2,3-二羧醛 anthracene-2,3-dicarbaldehyde 76197-35-8 C16H10O2 234.254
反应信息
-
作为反应物:描述:参考文献:名称:Sequential condensation and double desulfonylative cyclopropanation of 1,2-bis(sulfonylmethyl)arenes with 3-arylacroleins: access to biscyclopropane-fused tetralins摘要:使用高效的tBuOK催化剂,通过1,2-双(磺酰甲基)芳烃与3-芳基丙烯醛的序贯缩合和双去磺酰基环丙烷化反应,可以高产得到含有六个相邻立体中心的双环丙烷并联四氢萘。DOI:10.1039/d2ob02188a
-
作为产物:描述:参考文献:名称:Ried; Bodem, Chemische Berichte, 1956, vol. 89, p. 708,711摘要:DOI:
文献信息
-
A Unified Catalytic Asymmetric (4+1) and (5+1) Annulation Strategy to Access Chiral Spirooxindole‐Fused Oxacycles作者:Min Gao、Yanshu Luo、Qianlan Xu、Yukun Zhao、Xiangnan Gong、Yuanzhi Xia、Lin HuDOI:10.1002/anie.202105282日期:2021.9A unified catalytic asymmetric (N+1) (N=4, 5) annulation reaction of oxindoles with bifunctional peroxides has been achieved in the presence of a chiral phase-transfer catalyst (PTC). This general strategy utilizes peroxides as unique bielectrophilic four- or five-atom synthons to participate in the C−C and the subsequent umpolung C−O bond-forming reactions with one-carbon unit nucleophiles, thus providing
-
Aerobic Lactonization of Diols by Biomimetic Oxidation作者:Yoshinori Endo、Jan-E. BäckvallDOI:10.1002/chem.201102168日期:2011.11.4Coming up for air: Highly efficient aerobic lactonization can be carried out by a biomimetic oxidation system based on coupled redox catalysts (ruthenium catalyst and electron transfer mediators). This system leads to a low‐energy electron transfer from diol to molecular oxygen. Various diols were aerobically oxidized to the corresponding five‐ to nine‐membered lactones in good to high yields under空气进入:高效的好氧内酯化反应可以通过基于偶合氧化还原催化剂(钌催化剂和电子传递介体)的仿生氧化系统进行。该系统导致低能电子从二醇转移到分子氧。在温和的反应条件下,各种二醇被好氧氧化成相应的五元至九元内酯(见方案)。
-
Synthesis of large ring 3,4-alkylenedioxythiophenes (ADOT) derivatives via Mitsunobu reaction作者:Zhaochao Xu、Joo-Hee Kang、Fang Wang、Seung-Min Paek、Seong-Ju Hwang、Youngmee Kim、Sung-Jin Kim、Jin-Ho Choy、Juyoung YoonDOI:10.1016/j.tetlet.2011.03.062日期:2011.64-butylenedioxy)thiophene (BuDOT). In this Letter, we use Mitsunobu reaction to synthesize a series of 3,4-alkylenedioxythiophenes (ADOTs) derivatives with 8- to 16-membered rings. The eight-membered compounds were obtained in high or excellent yield. We also found that the 9- to 16-membered EDOT analogs were obtained in relatively low yield because of the competitive reaction to make dimers. Our method provides an easy聚(3,4-乙撑二氧噻吩)(PEDOT)以其优化的电导率,稳定性和高度透明性而著称,这使其成功实现了商业化。PEDOT的这些优异性能主要归因于3位和4位的亚烷基二氧基桥,因此,人们在合成3,4-乙撑二氧噻吩(EDOT)类似物上付出了很多努力。但是,只有很少的同源化合物能够成功合成,例如3,4-丙烯二氧噻吩(PrDOT)或3,4-(1,4-丁烯二氧基)噻吩(BuDOT)。在这封信中,我们使用Mitsunobu反应合成了一系列具有8至16元环的3,4-亚烷基二氧噻吩(ADOT)衍生物。以高收率或优异的收率获得了八元化合物。我们还发现,由于竞争性反应可生成二聚体,因此以相对较低的产率获得了9至16元的EDOT类似物。我们的方法提供了一种简单的方法来修饰乙二氧基噻吩(EDOT),并且这些获得的ADOTs化合物有望成为合成材料化学中使用的功能性π-共轭体系的基础。
-
Cobalt-Catalyzed Formal [4+2] Cycloaddition of α,α′-Dichloro-<i>ortho</i>-Xylenes with Alkynes作者:Kimihiro Komeyama、Yuji Okamoto、Ken TakakiDOI:10.1002/anie.201406807日期:2014.10.13A formal [4+2] cycloaddition of α,α′‐dichloro‐ortho‐xylenes with various alkynes has been developed using a low‐valent cobalt catalyst. The transformation has a wide substrate scope and high functional‐group tolerance and led to 1,4‐dihydronaphthalenes. The formed cycloadducts were easily aromatized with MnO2 under air. A mechanistic investigation suggests that the transformation proceeds through a
-
Iterative synthesis of acenesvia homo-elongation作者:Chih-Hsiu Lin、Ke-Han Lin、Bikash Pal、Li-Der TsouDOI:10.1039/b814840f日期:——Starting from aromaticortho-dialdehydes, we devised a homo-elongation protocol that combines a Wittig olefination and subsequent intramolecular Knoevenagel condensation to produce acene diesters and dinitriles.从芳香邻二醛出发,我们设计了一套同系延长方案,结合了Wittig烯化反应和随后的分子内Knoevenagel缩合反应,以生成并五烷二酯和二腈。
表征谱图
-
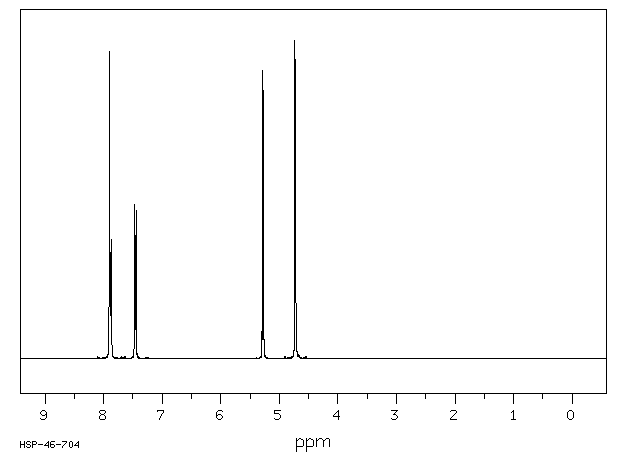
氢谱1HNMR
-
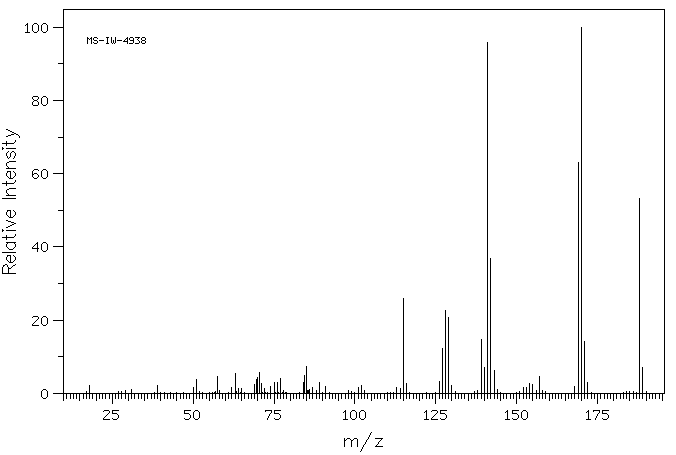
质谱MS
-
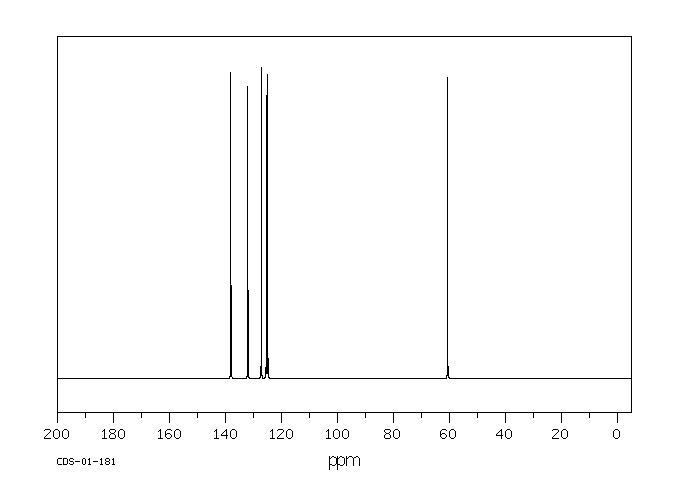
碳谱13CNMR
-
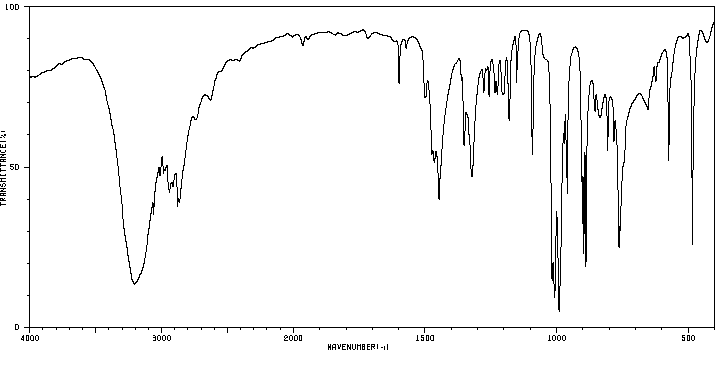
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮