2,5-二氯苯甲醇 | 34145-05-6
中文名称
2,5-二氯苯甲醇
中文别名
2,5-二氯苄醇
英文名称
2,5-dichlorobenzyl alcohol
英文别名
(2,5-dichlorophenyl)methanol
CAS
34145-05-6
化学式
C7H6Cl2O
mdl
MFCD00004607
分子量
177.03
InChiKey
LCEIGNVIDJNUGF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:79-80 °C (lit.)
-
沸点:266℃
-
密度:1.392
-
闪点:114℃
-
稳定性/保质期:
如果按照规定使用和储存,则不会分解,且未有已知危险反应。请避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2906299090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
| Name: | 2 5-Dichlorobenzyl Alcohol 99% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 34145-05-6 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 34145-05-6 | 2,5-Dichlorobenzyl Alcohol, 99% | 251-850-6 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
No information regarding eye irritation and other potential effects was found.
Skin:
No information regarding skin irritation and other potential effects was found.
Ingestion:
The toxicological properties of this substance have not been fully investigated.
Inhalation:
The toxicological properties of this substance have not been fully investigated.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use only in a well-ventilated area.
Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 34145-05-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: white
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 78 - 80 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H6Cl2O
Molecular Weight: 177.03
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Not available.
Incompatibilities with Other Materials:
Oxidizing agents, acids, acid chlorides, acid anhydrides.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 34145-05-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,5-Dichlorobenzyl Alcohol, 99% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 34145-05-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 34145-05-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 34145-05-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,5-二氯苯甲酸 2,5-dichlorobenzoic acid 50-79-3 C7H4Cl2O2 191.014 2,5-二氯苯甲醛 2,5-dichloro-benzaldehyde 6361-23-5 C7H4Cl2O 175.014 2,5-二氯苯甲基氯 1,4-Dichloro-2-chloromethyl-benzene 2745-49-5 C7H5Cl3 195.476 2,3-二氯苯甲酸甲酯 methyl 2,5-dichlorobenzoate 2905-69-3 C8H6Cl2O2 205.04 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,5-二氯苯甲醛 2,5-dichloro-benzaldehyde 6361-23-5 C7H4Cl2O 175.014 2,5-二氯苯甲基氯 1,4-Dichloro-2-chloromethyl-benzene 2745-49-5 C7H5Cl3 195.476 2,5-二氯苄基溴 2,5-dichlorobenzylbromide 85482-13-9 C7H5BrCl2 239.927
反应信息
-
作为反应物:参考文献:名称:Inhibitors of phenylethanolamine N-methyltransferase and epinephrine biosynthesis. 1. Chloro-Substituted 1,2,3,4-tetrahydroisoquinolines摘要:In a search for inhibitors of epinephrine biosynthesis as potential therapeutic agents, a series of 13 ring-chlorinated 1,2,3,4-tetrahydroisoquinolines was prepared. These compounds were tested initially for their ability to inhibit rabbit adrenal phenylethanolamine N-methyltransferase (PNMT) in vitro. Enzyme-inhibitor dissociation constants, determined for the six most potent members of the series, indicated the following order of decreasing potency: 7,8-Cl2 greater than 6,7,8-Cl3 greater than 7-Cl approximately 5,6,7,8-Cl4 greater than 5,7,8-Cl3. These compounds were subsequently examined for PNMT-inhibiting activity in intact rats and mice. 7,8-Dichloro-1,2,3,4-tetrahydroisoquinoline (13, SK&F 64139) was the most potent member of the series both in vitro and in vivo and is currently undergoing clinical investigation.DOI:10.1021/jm00179a007
-
作为产物:参考文献:名称:氢化锌控制的将羧酰胺还原为醇或胺摘要:使用氢化钠(NaH)和卤化锌(ZnX 2)的组合,建立了将羧酰胺控制还原为醇或胺的新方案。在ZnX 2上使用不同的卤化物决定了选择性,其中NaH-ZnI 2系统提供醇,而NaH-ZnCl 2系统提供胺。通过实验和理论方法进行的广泛机理研究表明,聚合氢化锌(ZnH 2)∞负责醇的形成,而二聚氯化锌氢化物(H-Zn-Cl)2是生产胺的关键物质。DOI:10.1002/anie.201900233
文献信息
-
TETRAZOLINONE COMPOUND AND USE OF SAME申请人:SUMITOMO CHEMICAL COMPANY, LIMITED公开号:US20160081339A1公开(公告)日:2016-03-24A tetrazolinone compound of formula (1): wherein R 1 and R 2 each independently represents a hydrogen atom, etc.; R 3 represents a C1-C6 alkyl group, etc.; R 4 , R 5 , and R 6 each independently represents a hydrogen atom, etc.; A represents a C6-C16 aryl group optionally having one or more atoms or groups selected from Group P, etc.; Q represents the following group Q1, etc.; and X represents an oxygen atom or a sulfur atom, has excellent control activity against pests.
-
Synthesis, in Vitro Pharmacology, Structure−Activity Relationships, and Pharmacokinetics of 3-Alkoxy-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic Acid Derivatives as Potent and Selective Group II Metabotropic Glutamate Receptor Antagonists作者:Atsuro Nakazato、Kazunari Sakagami、Akito Yasuhara、Hiroshi Ohta、Ryoko Yoshikawa、Manabu Itoh、Masato Nakamura、Shigeyuki ChakiDOI:10.1021/jm0400294日期:2004.8.1Novel group II metabotropic glutamate receptor (mGluR) antagonists, 3-alkoxy-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid derivatives 11 and 12, were discovered by the incorporation of a hydroxy or alkoxyl group onto the C-3 portion of selective and potent group II mGluR agonist 5, (1R,2S,5R,6R)-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid. Among these compounds, (1R,2R通过掺入羟基或烷氧基,发现了新型的II类代谢型谷氨酸受体(mGluR)拮抗剂3-烷氧基-2-氨基-6-氟双环[3.1.0]己烷-2,6-二羧酸衍生物11和12。在选择性和有效的第II组mGluR激动剂5(1R,2S,5R,6R)-2-氨基-6-氟双环[3.1.0]己烷-2,6-二羧酸的C-3部分上连接基团 在这些化合物中,(1R,2R,3R,5R,6R)-2-氨基-3-(3,4-二氯苄氧基)-6-氟双环[3.1.0]己烷-2,6-二羧酸(-)- 11be(MGS0039)是一种具有最佳药代动力学特征的高选择性强效II组mGluR拮抗剂。化合物(-)-11对mGlu 2(Ki = 2.38 +/- 0.40 nM)和mGlu 3(4.46 +/- 0.31 nM)表现出高亲和力,但对mGluR 7(Ki = 664 +/- 106 nM)的亲和力低,和有效的mGlu 2拮抗剂活性(IC50 =
-
[EN] (THIO)MORPHOLINE DERIVATIVES AS S1P MODULATORS<br/>[FR] DÉRIVÉS DE (THIO)MORPHOLINE MODULATEURS DE S1P申请人:ABBOTT HEALTHCARE PRODUCTS BV公开号:WO2011023795A1公开(公告)日:2011-03-03The present invention relates to (thio)morpholine derivatives of the formula (I), wherein R1 is selected from cyano, (2-4C)alkynyl, (1-4C)alkyl, (3-6C)cycloalkyl, (4-6C)cycloalkenyl, (6-8C)bicycloalkyl, (8-10C)bicyclic group, each optionally substituted with (1-4C)alkyl, phenyl, biphenyl, naphthyl, each optionally substituted with one or more substituents independently selected from halogen, (1-4C)alkyloptionally substituted with one or more fluoro atoms, (2-4C)alkynyl, (1-4C)alkoxy optionally substituted with one or more fluoro atoms,amino, di(1-4C)alkylamino, -SO2-(1-4C)alkyl, -CO-(1-4C)alkyl, -CO-O-(1-4C)alkyl, -NH-CO-(1-4C)alkyl and (3-6C)cycloalkyl, phenyl substituted with phenoxy, benzyl, benzyloxy, phenylethyl or monocyclic heterocycle, each optionally substituted with (1-4C)alkyl, monocyclic heterocycle optionally substituted with halogen, (1-4C)alkyl or with phenyl optionally substituted with (1-4C)alkyl, and bicyclic heterocycle optionally substituted with (1-4C)alkyl; A is selected from -CO-O-, -O-CO-, -NH-CO-, -CO-NH, -C=C-, -CCH3-O- and the linking group –Y-(CH2)n-X- wherein Y is attached to R1 and selected from a bond, -O-, -S-, -SO-, -SO2-, -CH2-O-, -CO-, -O-CO-, -CO-O-, -CO-NH-, -NH-CO-, -C=C-and -C≡C-; n is an integer from 1 to 10; and X is attached to the phenylene / pyridyl group and selected from a bond, -O-, -S-, -SO-, -SO2 -, -NH, -CO-, -C=C-and -C≡C-; ring structure B optionally contains one nitrogen atom; R2 is H, (1-4C)alkyl optionally substituted with one or more fluoro atoms, (1-4C)alkoxy optionally substituted with one or more fluoro atoms, or halogen; and R3 is (1-4C)alkylene-R5 wherein the alkylene group may be substituted with (CH2)2 to form a cyclopropyl moiety or one or two halogen atoms, or R3 is is (3-6C)cycloalkylene-R5 or -CO-CH2-R5, wherein R5 is -OH, -PO3H2, -OPO3H2, -COOH, -COO(1-4C)alkyl or tetrazol-5-yl; R4 is H or (1-4C)alkyl; R6 is one or more substituents independently selected from H, (1-4C)alkyl or oxo; W is -O-, -S-, -SO- or -SO2-; or a pharmaceutically acceptable salt, a solvate or hydrate thereof; with the proviso that the derivative of formula (I) is not 2-(4-ethylphenyl)-4-morpholinoethanol or 4-[4-(2-hydroxyethyl)-2-morpholinyl]benzeneacetonitrile or a pharmaceutically acceptable salt, a solvate or hydrate thereof. The compounds of the invention have affinity to S1P receptors and may be used in the treatment, alleviation or prevention of S1P receptor mediated diseases and conditions.本发明涉及公式(I)的(硫)吗啉衍生物,其中R1从氰基,(2-4C)炔基,(1-4C)烷基,(3-6C)环烷基,(4-6C)环烯基,(6-8C)双环烷基,(8-10C)双环基团中选择,每个基团可选择地取代为(1-4C)烷基,苯基,联苯基,萘基,每个基团可选择地取代为一个或多个取代基,独立选择自卤素,(1-4C)烷基可选择地取代为一个或多个氟原子,(2-4C)炔基,(1-4C)氧烷基可选择地取代为一个或多个氟原子,氨基,二(1-4C)烷基氨基,-SO2-(1-4C)烷基,-CO-(1-4C)烷基,-CO-O-(1-4C)烷基,-NH-CO-(1-4C)烷基和(3-6C)环烷基,苯基取代为苯氧基,苄基,苄氧基,苯乙基或单环杂环烃,每个基团可选择地取代为(1-4C)烷基,单环杂环烃可选择地取代为卤素,(1-4C)烷基或取代为苯基的苯基,可选择地取代为(1-4C)烷基,和双环杂环烃可选择地取代为(1-4C)烷基;A从-CO-O-,-O-CO-,-NH-CO-,-CO-NH,-C=C-,-CCH3-O-和连接基-Y-(CH2)n-X-中选择,其中Y连接到R1并从键,-O-,-S-,-SO-,-SO2-,- -O-,-CO-,-O-CO-,-CO-O-,-CO-NH-,-NH-CO-,-C=C-和-C≡C-中选择;n是1到10的整数;X连接到苯基/吡啶基团并从键,-O-,-S-,-SO-,-SO2-,-NH,-CO-,-C=C-和-C≡C-中选择;环结构B可选择地含有一个氮原子;R2是H,(1-4C)烷基可选择地取代为一个或多个氟原子,(1-4C)氧烷基可选择地取代为一个或多个氟原子,或卤素;R3是(1-4C)烷基-R5,其中烷基基团可取代为( )2形成环丙基基团或一个或两个卤素原子,或R3是(3-6C)环烷基-R5或-CO- -R5,其中R5是-OH,-PO3H2,-OPO3H2,-COOH,-COO(1-4C)烷基或四唑-5-基;R4是H或(1-4C)烷基;R6是一个或多个取代基,独立选择自H,(1-4C)烷基或氧代基;W是-O-,-S-,-SO-或-SO2-;或其药学上可接受的盐,溶剂或水合物;但是,公式(I)的衍生物不是2-(4-乙基苯基)-4-吗啉乙醇或4-[4-(2-羟乙基)-2-吗啉基]苯乙腈或其药学上可接受的盐,溶剂或水合物。本发明的化合物具有对S1P受体的亲和力,可用于治疗、缓解或预防S1P受体介导的疾病和症状。
-
Hydrogenation of Esters to Alcohols Catalyzed by Defined Manganese Pincer Complexes作者:Saravanakumar Elangovan、Marcel Garbe、Haijun Jiao、Anke Spannenberg、Kathrin Junge、Matthias BellerDOI:10.1002/anie.201607233日期:2016.12.5The first manganese‐catalyzed hydrogenation of esters to alcohols has been developed. The combination of Mn(CO)5Br with [HN(CH2CH2P(Et)2)2] leads to a mixture of cationic and neutral Mn PNP pincer complexes, which enable the reduction of various ester substrates, including aromatic and aliphatic esters as well as diesters and lactones. Notably, related pincer complexes with isopropyl or cyclohexyl
-
Efficient Difluoromethylation of Alcohols Using TMSCF<sub>2</sub>Br as a Unique and Practical Difluorocarbene Reagent under Mild Conditions作者:Qiqiang Xie、Chuanfa Ni、Rongyi Zhang、Lingchun Li、Jian Rong、Jinbo HuDOI:10.1002/anie.201611823日期:2017.3.13difluoromethylation of alcohols using commercially available TMSCF2Br (TMS=trimethylsilyl) as a unique and practical difluorocarbene source is developed. This method allows primary, secondary, and even tertiary alkyl difluoromethyl ethers to be synthesized under weakly basic or acidic conditions. The reaction mainly proceeds through the direct interaction between a neutral alcohol and difluorocarbene
表征谱图
-
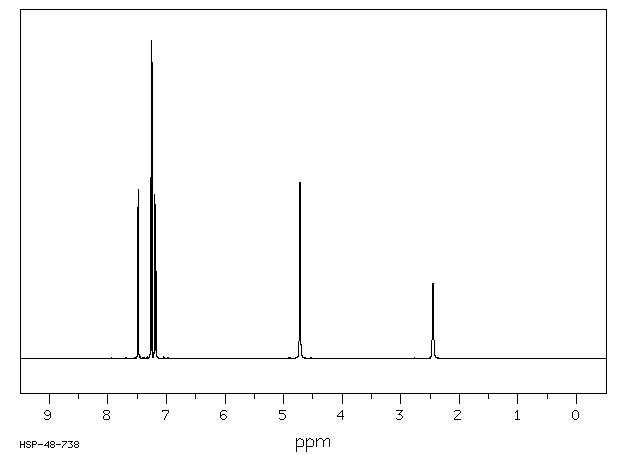
氢谱1HNMR
-
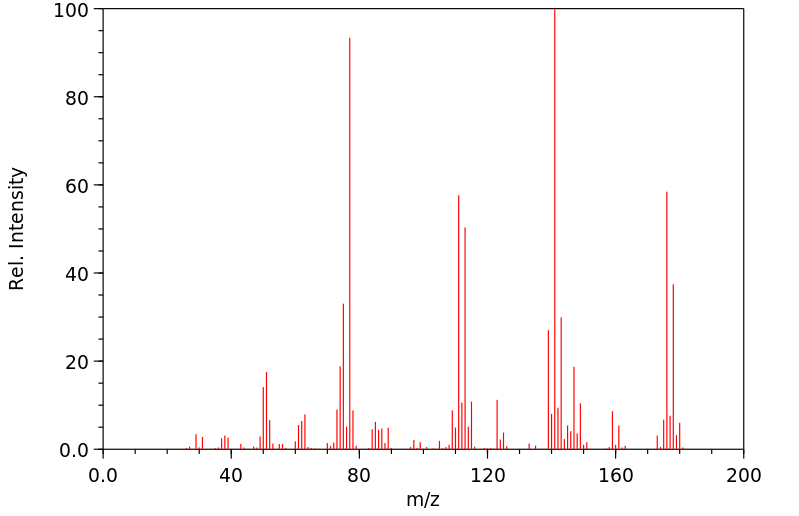
质谱MS
-
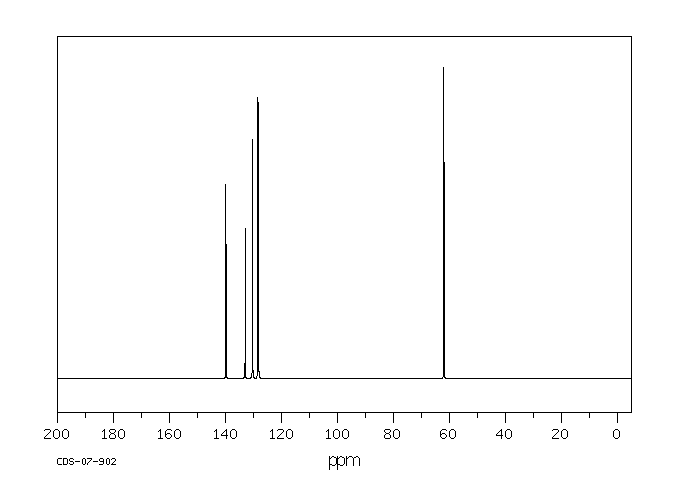
碳谱13CNMR
-
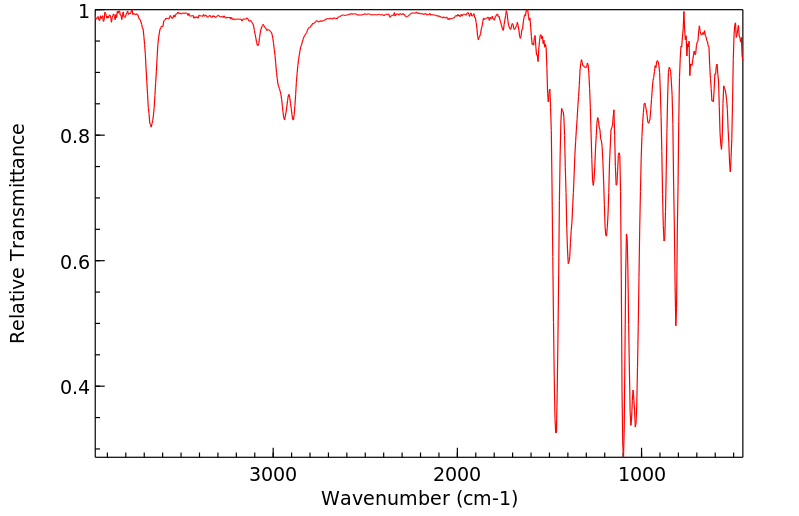
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫