2,6-二甲基-3,5-二(苯基)吡喃-4-酮 | 33731-54-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:21
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2932999099
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3,5-diphenyl-2-formyl-6-methyl-4H-pyran-4-one 500716-71-2 C19H14O3 290.318 —— 2,6-diformyl-3,5-diphenyl-4H-pyran-4-one 500716-73-4 C19H12O4 304.302 —— 2,6-Bis(brommethyl)-3,5-diphenyl-4-pyranon 124945-37-5 C19H14Br2O2 434.127 —— 6-bromomethyl-2-dibromomethyl-3,5-diphenyl-4H-pyran-4-one —— C19H13Br3O2 513.023 —— 16,18-Diphenyl-2,11-dithia<3>metacyclo<3>(2,6)pyranophan-17-on 124945-41-1 C27H22O2S2 442.602 —— 2,6-dimethyl-3,5-diphenyl-4H-pyran-4-thione 73499-29-3 C19H16OS 292.401
反应信息
-
作为反应物:描述:2,6-二甲基-3,5-二(苯基)吡喃-4-酮 在 selenium(IV) oxide 作用下, 以 1,4-二氧六环 为溶剂, 反应 32.0h, 以65%的产率得到3,5-diphenyl-2-formyl-6-methyl-4H-pyran-4-one参考文献:名称:Habibi; Bayat; Marandi, Asian Journal of Chemistry, 2012, vol. 24, # 11, p. 5239 - 5241摘要:DOI:
-
作为产物:参考文献:名称:意外的4,5-二苯基-2,7-萘啶衍生物具有聚集诱导的发射和从3,5-二苯基-4H-吡喃衍生物获得的机械荧光色性。摘要:对于特定的荧光分子,分子构象畸变的增加有利于赋予其聚集诱导发射(AIE)和机械荧光致变色(MFC)特性。本文合成了具有高度扭曲构象的3,5-二苯基-4 H-吡喃衍生物5和4,5-二苯基-2,7-萘啶衍生物7。对于化合物5,尽管在4 H-吡喃骨架的3和5位引入具有大位阻的苯环实现了从聚集诱导猝灭(ACQ)活性分子到AIE活性分子的转变,但仅显示了低对比度MFC活动。化合物7被意外地从化合物获得5和正丁胺通过开环和随后的分子内闭环机理。通过晶体结构分析确认化合物7具有高度扭曲的分子构象,并且显示出源自分子内旋转的限制的AIE活性。此外,化合物7具有可逆的高对比度MFC活性。研磨后,确认固态荧光颜色从橙色变为黄色,这是由于晶体结构的部分破坏所致。这项工作为基于ACQ活性母体分子的新型AIE活性和MFC活性荧光分子的设计和合成提供了新思路。DOI:10.1002/asia.202000884
文献信息
-
The Acid-catalyzed Reaction of the 2-Oxabicyclo[4.1.0]hept-3-en-5-one System: Isomerization from Homo-4-pyrones into 2-Furylacetone Derivatives作者:Hiroshi Yamaoka、Ikuhiro Mishima、Mitsuko Miyamoto、Terukiyo HanafusaDOI:10.1246/bcsj.53.469日期:1980.2monocyclopropanation of 4-pyrones with dimethyloxosulfonium methylide, using a dimethyl sulfoxide hexamethylphosphoric triamide medium in place of neat dimethyl sulfoxide, gave homo-4-pyrone derivatives, which were then rearranged into the 2-furylacetone derivatives, along with hydra ted triketones or the dehydrated naphtho[2,1-b]furan derivative, by means of strong acid catalysis at room temperature.
-
Synthesis of Novel Imidazolium and Benzimidazolium Salts from Halomethyl Derivatives of 4-Pyrones作者:Zarrin Ghasemi、Mahdi Gholamhoseini-Nazari、Maryam Allahvirdinesbat、Mahnaz Saraei、Aziz ShahrisaDOI:10.2174/157017812803521153日期:2012.10.1A series of mono- and bis-benzimidazolium and imidazolium salts of 4-pyrone derivatives has been synthesized in good yields, by various quaternization reactions of halomethyl derivatives of 4H-pyran-4-ones. These salts can be used as novel precursors of functionalized N-heterocyclic carbenes and some of them as novel ionic liquids.
-
An efficient synthesis of carboxaldehyde derivatives of 4<i>H</i>-pyran-4-one作者:Reza Teimuri-mofrad、Fatemeh AbrishamiDOI:10.1139/v07-034日期:2007.5.1
We developed a general method for the synthesis of various 2-mono- and 2,6-di-carboxaldehyde substituted derivatives of 3,5-diphenyl-4H-pyran-4-one and 4H-pyran-4-one. 3,5-Diphenyl-6-methyl-4-oxo-4H-pyran-2-carboxaldehyde (4a), 6-methyl-4-oxo-4H-pyran-2-carboxaldehyde (4b), 3,5-diphenyl-4-oxo-4H-pyran-2,6-dicarboxaldehyde (5a), 4-oxo-4H-pyran-2,6-dicarboxaldehyde (5b), 3,5-diphenyl-6-hydroxymethyl-4-oxo-4H-pyran-2-carboxaldehyde (10a), and 6-hydroxymethyl-4-oxo-4H-pyran-2-carboxaldehyde (10b) were obtained from the corresponding di-, tri-, and tetra-bromo derivatives of 2,6-dimethyl-3,5-diphenyl-4H-pyran-4-one (1a) and 2,6-dimethyl-4H-pyran-4-one (1b) by treatment with silver acetate followed by hydrolysis. Compounds 4a and 4b were also obtained by the oxidation of 10a and 10b with barium manganate.Key words: 4H-pyran-4-one, hydroxymethyl and carboxaldehyde derivatives, acetoxylation, hydrolysis, oxidation.
我们开发了一种合成 3,5-二苯基-4H-吡喃-4-酮和 4H-吡喃-4-酮的各种 2-单甲醛和 2,6-二甲醛取代衍生物的通用方法。3,5-Diphenyl-6-methyl-4-oxo-4H-pyran-2-carboxaldehyde (4a), 6-methyl-4-oxo-4H-pyran-2-carboxaldehyde (4b), 3,5-diphenyl-4-oxo-4H-pyran-2,6-二甲醛 (5a)、4-氧代-4H-吡喃-2,6-二甲醛 (5b)、3,5-二苯基-6-羟甲基-4-氧代-4H-吡喃-2-甲醛 (10a)、和 6-羟甲基-4-氧代-4H-吡喃-2-甲醛(10b)是由 2,6-二甲基-3,5-二苯基-4H-吡喃-4-酮(1a)和 2,6-二甲基-4H-吡喃-4-酮(1b)的相应二溴、三溴和四溴衍生物经乙酸银处理后水解得到的。化合物 4a 和 4b 也是通过 10a 和 10b 与锰酸钡的氧化反应得到的:4H-吡喃-4-酮、羟甲基和甲醛衍生物、乙酰氧基化、水解、氧化。 -
有机磷光材料及其制备方法和防伪油墨
-
The Formation of Two Kinds of Thiophene Derivatives by the Reaction of the 4<i>H</i>-Pyran-4-thione Derivative with Dimethyloxosulfonium Methylide in a One-pot Process作者:Hiroshi Yamaoka、Ikuhiro Mishima、Terukiyo HanafusaDOI:10.1246/bcsj.53.1763日期:1980.6The reaction of 2,6-dimethyl-3,5-diphenyl-4H-pyran-4-thione with dimethyloxosulfonium methylide occurred facilely to give two kinds of 2-thienylacetones, accompanied by a five-membered α,β-unsaturated thione, a naphtho-[2,1-b]thiophene derivative, and a hemithioacetal.
表征谱图
-
氢谱1HNMR
-
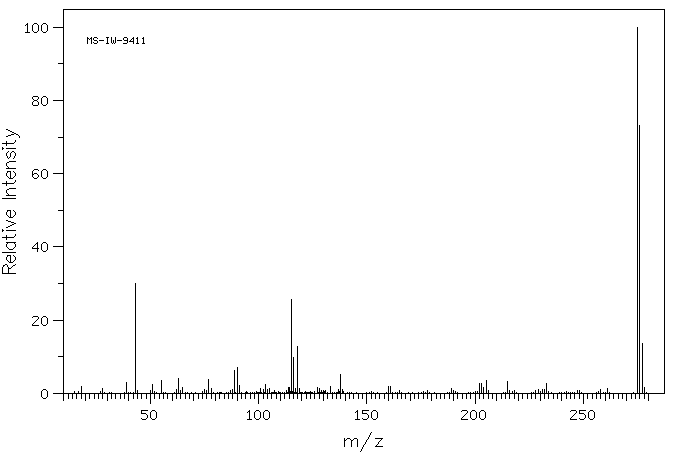
质谱MS
-
碳谱13CNMR
-
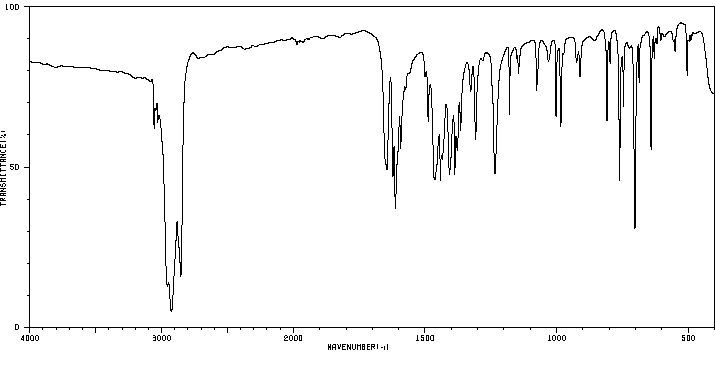
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息