2,6-双(4-氟代苯亚甲基)环己酮 | 395-12-0
中文名称
2,6-双(4-氟代苯亚甲基)环己酮
中文别名
2,6-双(4-氟代亚苄)环己酮
英文名称
(2E,6E)-2,6-bis(4-fluorobenzylidene)cyclohexanone
英文别名
(2E, 6E)−2,6-bis(4-fluorobenzylidene)cyclohexanone;2,6-bis((E)-4-fluorobenzylidene)cyclohexan-1-one;2,6-[(E,E)-bis-(4-fluoro-benzylidene)]-cyclohexanone;2,6-[(E,E)-Bis-(4-fluor-benzyliden)]-cyclohexanon;2,6-Bis(4-fluorobenzylidene)cyclohexanone;(2E,6E)-2,6-bis[(4-fluorophenyl)methylidene]cyclohexan-1-one
CAS
395-12-0;62085-74-9
化学式
C20H16F2O
mdl
——
分子量
310.343
InChiKey
STPNBQOTKFPYEG-UNZYHPAISA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:147-154 °C
计算性质
-
辛醇/水分配系数(LogP):5.3
-
重原子数:23
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.15
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为反应物:描述:2,6-双(4-氟代苯亚甲基)环己酮 在 盐酸羟胺 、 sodium acetate 作用下, 以 乙醇 、 水 为溶剂, 以45%的产率得到2-[(4-Fluoro-phenyl)-hydroxyamino-methyl]-6-[1-(4-fluoro-phenyl)-meth-(E)-ylidene]-cyclohexanone oxime参考文献:名称:一些6-亚芳基-2-(α-羟基氨基-α-芳基甲基)环己酮肟及其相关化合物的合成和细胞毒性评估。摘要:2,6-双-(苯基亚甲基)环己酮(1)与4摩尔过量的羟胺盐酸盐和乙酸钠反应生成相应的肟2,生成2-(α-羟基氨基-α-苯基甲基)-6-苯基亚甲基环己酮一肟(5a),其结构由高分辨率质子核磁共振波谱推导,并通过X射线分析证实。最终由1与羟胺本身而不是由盐酸羟胺和乙酸钠的混合物制备化合物2。制备了10a的5a类似物,即5b-5k,并评估了细胞毒性。第5系列和11系列的11种化合物中有6种在240-950 microM范围内具有抗鼠乳腺EMT6细胞的活性。DOI:10.1002/jps.2600811103
-
作为产物:描述:参考文献:名称:Synthesis and Anti-bacterial Properties of Mono-carbonyl Analogues of Curcumin摘要:本文描述了三种单羰基姜黄素类似物的合成。对七种革兰氏阳性和革兰氏阴性细菌进行了体外抗菌活性测试,并讨论了取代基对芳环和连接张力的空间结构的影响。观察到,与姜黄素相比,部分衍生物显示出显著的活性,并且大多数衍生物对氨苄西林抵抗的阴沟肠杆菌表现出活性。化合物A12、B09、B13、B14和C09显示出显著的体外抗菌活性。结果显示,杂环或长链取代基可能增强姜黄素类似物的活性。DOI:10.1248/cpb.56.162
文献信息
-
[EN] CURCUMIN ANALOGS AND METHODS OF USE THEREOF<br/>[FR] ANALOGUES DE LA CURCUMINE ET PROCÉDÉS D'UTILISATION申请人:UNIV RUTGERS公开号:WO2012021692A1公开(公告)日:2012-02-16Curcumin analogs and methods of use thereof are provided.提供了姜黄素类似物及其使用方法。
-
Antiparasitic activity of synthetic curcumin monocarbonyl analogues against Trichomonas vaginalis作者:Caroline Carapina da Silva、Bruna Silveira Pacheco、Raquel Nascimento das Neves、Mirna Samara Dié Alves、Ângela Sena-Lopes、Sidnei Moura、Sibele Borsuk、Claudio Martin Pereira de PereiraDOI:10.1016/j.biopha.2018.12.058日期:2019.3herein we report the anti-T. vaginalis evaluation of 21 synthetic monocarbonyl analogues of curcumin, which itself has been reported to possess antiparasitic potential. From the in vitro analysis of the synthetic molecules, untreated trophozoites, and metronidazole at 100 μM, it was observed that three curcumin analogues (3a, 3e, and 5e) exhibited anti-T. vaginalis activity comparable to metronidazole毛滴虫病是由阴道毛滴虫引起的寄生虫感染,被认为是世界上最常见的非病毒性传播感染。自1960年代以来,硝基咪唑类(如甲硝唑)是治疗滴虫的首选药物,但使用它们可能会导致许多不良反应和过敏反应。甲硝唑耐药性感染的报告也突出了寻找新的抗T抗体的重要性。阴道剂。考虑到这一点,我们在此报告抗-T。对姜黄素的21种合成单羰基类似物进行阴道评估,据报道其本身具有抗寄生虫潜力。从合成的分子,未处理的滋养体和甲硝唑在100μM的体外分析中,观察到三种姜黄素类似物(3a,3e和5e)均显示抗T。阴道活性与甲硝唑相当(无统计学差异)。丙酮衍生物3a和3e的最佳抗寄生虫浓度分别为80μM和90μM,环己酮衍生物5e的最佳抗寄生虫浓度为200μM。动力学生长曲线表明,在24小时后,滋养体被完全抑制。在测试浓度下,天然姜黄素并未显着抑制滋养体的生长,因此表明所设计的合成分子不仅具有更好的化学稳定性,而且具有更高的抗T活
-
Ag/P-Stereogenic Phosphine-Catalyzed Enantioselective 1,3-Dipolar Cycloadditions: A Method to Optically Active Pyrrolidines作者:Mengna Zhi、Zhenjie Gan、Rong Ma、Hao Cui、Er-Qing Li、Zheng Duan、François MatheyDOI:10.1021/acs.orglett.9b00926日期:2019.5.3A Ag/P-stereogenic phosphine-complex-catalyzed 1,3-dipolar cycloaddition of azomethine ylides with electron-deficient olefins is reported. In this reaction, highly functionalized pyrrolines with a spiro-quaternary stereogenic center were obtained in good yields (up to 99%) with excellent levels of diastereo- (up to >20:1 dr) and enantioselectivities (up to >99% ee). The chirality of adducts was controlled
-
Curcumin-like diarylpentanoid analogues as melanogenesis inhibitors作者:Takahiro Hosoya、Asami Nakata、Fumie Yamasaki、Faridah Abas、Khozirah Shaari、Nordin Hj Lajis、Hiroshi MoritaDOI:10.1007/s11418-011-0568-0日期:2012.1Anti-melanogenesis screening of 47 synthesized curcumin-like diarylpentanoid analogues was performed to show that some had a potent inhibitory effect on the melanogenesis in B16 melanoma cells. Their actions were considered to be mostly due to tyrosinase inhibition, tyrosinase expression inhibition, and melanin pigment degradation. The structure–activity relationships of those curcumin-like diarylpentanoid analogues which inhibited the melanogenesis and tyrosinase activity were also discussed. Of those compounds assayed, (2E,6E)-2,6-bis(2,5-dimethoxybenzylidene)cyclohexanone showed the most potent anti-melanogenesis effect, the mechanism of which is considered to be the degradation of the melanin pigment in B16 melanoma cells, affecting neither the tyrosinase activity nor tyrosinase expression.
-
Symmetrical and unsymmetrical substituted 2,5-diarylidene cyclohexanones as anti-parasitic compounds作者:Zia Ud Din、Marilia Almeida Trapp、Lívia Soman de Medeiros、Danielle Lazarin-Bidóia、Francielle Pelegrin Garcia、Francieli Peron、Celso Vataru Nakamura、Ihosvany Camps Rodríguez、Abdul Wadood、Edson Rodrigues-FilhoDOI:10.1016/j.ejmech.2018.06.031日期:2018.7epimastigoteand trypomastigoteof Trypanosoma cruzi. Eighteen compounds displayed anti-leishmanial activity against promastigotes of L. amazonensis with IC50 values ranging from 2.8 to 10 μM. In addition, two compounds exhibited significant antitrypanosomal activity against epimastigotes of T. cruzi with IC50 values of 5.2 ± 0.8 and 3.0 ± 0.0 μM, while five compounds exhibited activity from 15.0 ± 1.4 to 30.2 ± 1通过用各种醛处理环己酮,以中等至极好的收率合成了对称和不对称的双芳基-α,β-不饱和酮。二甲基氨基甲酸二甲基铵(DIMCARB)既用作催化剂,又用作反应介质,用于合成单亚芳基环加合物中间体,该中间体还用于生产二亚芳基环己酮。评估了所有34种合成化合物的抗增殖活性,特别是针对亚马逊利什曼原虫的前鞭毛体,克氏锥虫的前鞭毛体和锥鞭毛体。十八种化合物对L的前鞭毛体显示出抗利什曼活性。亚马逊IC 50值范围从2.8到10μM。另外,两种化合物表现出对的epimastigotes显著抗锥体虫活动锥虫带IC 50为5.2±0.8和3.0±0.0μM值,而五种化合物表现出的活性为15.0±1.4 30.2±1.8μM针对的锥鞭毛体克氏锥虫。而且,与上皮细胞相比,所有化合物对寄生虫的选择性更高。不对称化合物16,28,30和33可以被认为是具有IC有利抗寄生虫先导分子50和EC 50值在低微摩尔范围内,优于
表征谱图
-
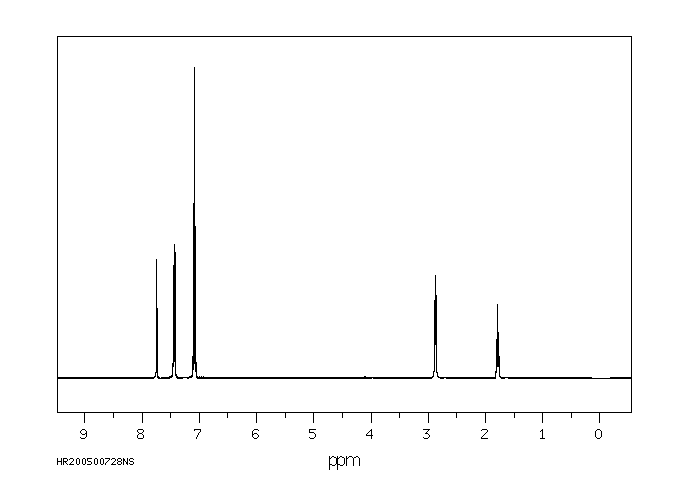
氢谱1HNMR
-
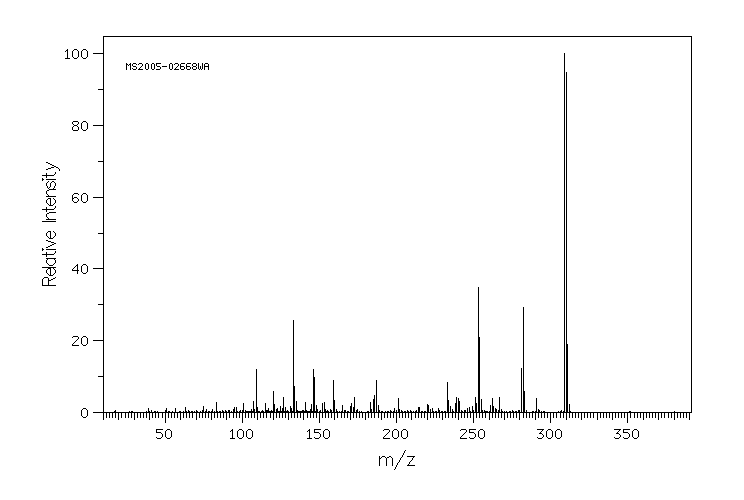
质谱MS
-
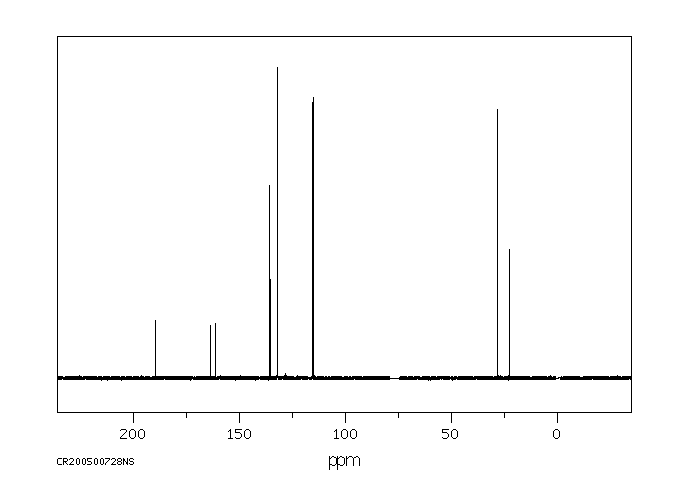
碳谱13CNMR
-
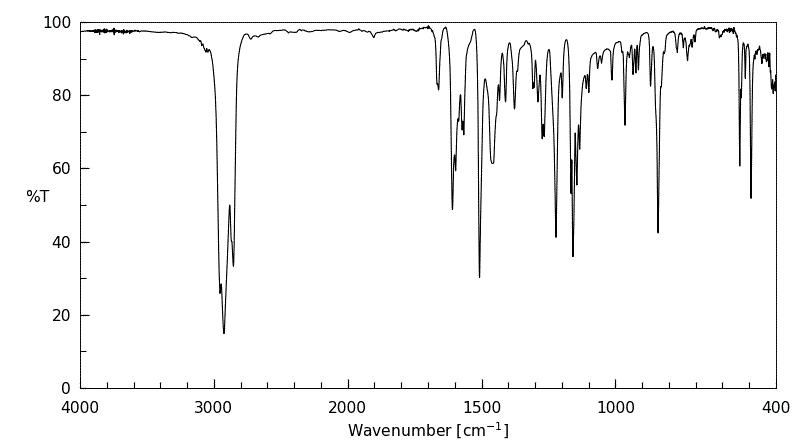
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(11aR)-3,7-双(3,5-二甲基苯基)-10,11,12,13-四氢-5-羟基-5-氧化物-二茚基[7,1-de:1'',7''-fg][1,3,2]二氧杂膦酸
龙血素C
顺-1,7-二苯基-1-庚烯基-5-醇
那洛西芬
赤杨酮
赤杨二醇
血竭素
蒙桑酮C
萘-2,7-二磺基酸,钠盐
苯酚,4-(1,3-二苯基丁基)-2-(1-苯基乙基)-
苯甲酸,2-[[2-[(2-羧基苯基)氨基]-5-(三氟甲基)苯基]氨基]-5-[[[(4-羟基-3-甲氧苯基)甲基]氨基]甲基]-
苯基-[4-(2-苯基乙炔基)苯基]甲酮
苯基-[2-[3-(三氟甲基)苯基]苯基]甲酮
苯基-[2-(2-苯基苯基)苯基]甲酮
苯基-(3-苯基萘-2-基)甲酮
苯基-(2-苯基环己基)甲酮
苯,[(二甲基苯基)甲基]甲基[(甲基苯基)甲基]-
苯,1,3-二[1-甲基-1-[4-(4-硝基苯氧基)苯基]乙基]-
脱甲氧姜黄
紫外吸收剂 234
粗糠柴苦素
硫酸姜黄素
矮紫玉盘素
益智醇
白桦林烯酮;1,7-双(4-羟基苯基)-4-庚烯-3-酮
甲酮,苯基(1,6,7,8-四氢-1-甲基-5-苯基环戊二烯并[g]吲哚-3-基)-
甲酮,[3-(4-甲氧苯基)-1-苯基-9H-芴-4-基]苯基-
甲酮,(4-氯苯基)[1-(4-氯苯基)-3-苯基-9H-芴-4-基]-
环香草酮
溴敌隆
波森
桤木酮
桑根酮D
杨梅醇
杨梅酮
杨梅联苯环庚醇-15-葡糖苷
替拉那韦
替吡法尼(S型对映体)
替吡法尼
曲沃昔芬
姜黄素葡糖苷酸
姜黄素beta-D-葡糖苷酸
姜黄素4,4'-二乙酸酯
姜黄素-d6
姜黄素
姜烯酮 A
奈帕芬胺杂质D
四甲基姜黄素
四氢脱甲氧基二阿魏酰甲烷
四氢姜黄素二乙酸酯