2-(2-甲基-1,3-二噁烷-2-基)乙酸乙酯 | 90293-83-7
中文名称
2-(2-甲基-1,3-二噁烷-2-基)乙酸乙酯
中文别名
——
英文名称
2-<(ethoxycarbonyl)methyl>-2-methyl-1,3-dioxane
英文别名
methyl-2 dioxanne-1,3 acetate-2 d'ethyle;2-Methyl-2-aethoxycarbonylmethyl-1,3-dioxan;(2-methyl-[1,3]dioxan-2-yl)-acetic acid ethyl ester;(2-Methyl-[1,3]dioxan-2-yl)-essigsaeure-aethylester;Ethyl 2-(2-methyl-1,3-dioxan-2-yl)acetate
CAS
90293-83-7
化学式
C9H16O4
mdl
——
分子量
188.224
InChiKey
XMLLBDKQPQZGDP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:13
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.89
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-(2-Methyl-1,3-dioxan-2-yl)acetic acid 188406-87-3 C7H12O4 160.17
反应信息
-
作为反应物:描述:参考文献:名称:Three- and Four-Carbon Elongating Ring Expansion of Cyclic Acetals to Medium-Sized Dioxacycloalkenones. Use of the Intramolecular Formation of Oxonium Ylides摘要:The Rh(II)-catalyzed reaction of 2-(3'-diazo-2'-oxopropyl)-2-methyldioxolane (1) in the presence of a protic nucleophile (NuH) such as AcOH resulted in effective ring enlargement to give the 8-membered 3-acetoxydioxocanone 4a (41%) and dioxocan-2-en-1-one 3 (46%). Similar treatment of 2-(4'-diazo-3'-oxobutyl)-2-methyldioxolane (9) with AcOH gave 4-acetoxydioxonanone 10 (67%), which was readily hydrolyzed on silica gel to a tautomeric mixture of hydrolysis products 16a and 16b (total yield 46%). In contrast, similar treatment of 2-(5'-diazo-4'-oxopentyl)-2-methyldioxolane (19) gave 2,5-dioxa-1-methyldecalin-7-one (20, 24%), and the yield increased to 61% in the absence of AcOH, by Stevens rearrangement. The reaction of 1,3-dioxane homologues 26 and 31 gave similar results. All of these reactions can be explained in terms of the intermediacy of bicyclooxonium ylides, which undergo either a Stevens rearrangement or, after protonation by a NuH, ring enlargement through release of the strain of the bicyclic ylides. Evidence of the reversible formation of oxonium ylides is also provided.DOI:10.1021/jo961844x
-
作为产物:描述:参考文献:名称:Three- and Four-Carbon Elongating Ring Expansion of Cyclic Acetals to Medium-Sized Dioxacycloalkenones. Use of the Intramolecular Formation of Oxonium Ylides摘要:The Rh(II)-catalyzed reaction of 2-(3'-diazo-2'-oxopropyl)-2-methyldioxolane (1) in the presence of a protic nucleophile (NuH) such as AcOH resulted in effective ring enlargement to give the 8-membered 3-acetoxydioxocanone 4a (41%) and dioxocan-2-en-1-one 3 (46%). Similar treatment of 2-(4'-diazo-3'-oxobutyl)-2-methyldioxolane (9) with AcOH gave 4-acetoxydioxonanone 10 (67%), which was readily hydrolyzed on silica gel to a tautomeric mixture of hydrolysis products 16a and 16b (total yield 46%). In contrast, similar treatment of 2-(5'-diazo-4'-oxopentyl)-2-methyldioxolane (19) gave 2,5-dioxa-1-methyldecalin-7-one (20, 24%), and the yield increased to 61% in the absence of AcOH, by Stevens rearrangement. The reaction of 1,3-dioxane homologues 26 and 31 gave similar results. All of these reactions can be explained in terms of the intermediacy of bicyclooxonium ylides, which undergo either a Stevens rearrangement or, after protonation by a NuH, ring enlargement through release of the strain of the bicyclic ylides. Evidence of the reversible formation of oxonium ylides is also provided.DOI:10.1021/jo961844x
文献信息
-
Bioinspired Collective Syntheses of Iboga-Type Indole Alkaloids作者:Gaoyuan Zhao、Xingang Xie、Haiyu Sun、Ziyun Yuan、Zhuliang Zhong、Shouchu Tang、Xuegong SheDOI:10.1021/acs.orglett.6b00989日期:2016.5.20We present the application of a bioinspired collective synthesis strategy in the total syntheses of seven iboga-type indole alkaloids: (±)-tabertinggine, (±)-ibogamine, (±)-ibogaine, (±)-ibogaine hydroxyindolenine, (±)-3-oxoibogaine hydroxyindolenine, (±)-iboluteine, and (±)-ervaoffines D. In particular, tabertinggine and its congeners serve as iboga precursors for the subsequent biomimetic transformations
-
Synthese electrochimique de methoxy-2 dioxa-1,4 cyclanes par oxydation anodique de cetals cycliques de β-ceto-carboxylates作者:Daniel Lelandais、Cathy Bacquet、Jacques EinhornDOI:10.1016/s0040-4020(01)98845-3日期:1981.1Anodic oxidation of β-oxocarboxylate cyclic acetals in anhydrous methanol gives 2-methoxy-1,4-diox-acycloalkanes in 40–60% yields. The mechanism is discussed.
-
Oxygen-containing hererocyclic compound, processes for their preparation and pharmaceutical compositions comprising them申请人:FUJISAWA PHARMACEUTICAL CO., LTD.公开号:EP0346511A1公开(公告)日:1989-12-20A compound of the formula : wherein R¹ is hydrogen or lower alkyl, R² is carboxy(lower)alkyl or protected carboxy(lower)alkyl and R³ is -CH₂NH-R⁴, -CH=N-R⁴ or -CH₂-R⁵ in which R⁴ is acyl, acylamino, heterocyclic amino, heterocyclic(lower)alkyl or ar(lower)alkoxy and R⁵ is acyloxy or heterocyclic(lower)alkoxy and X is -O- or -CH₂-, or pharmaceutically acceptable salt thereof, processes for their preparation and pharmaceutical compositions comprising them as an active ingredient.一种化合物的公式为:其中R¹为氢或较低的烷基,R²为羧基(较低)烷基或保护羧基(较低)烷基,R³为-CH₂NH-R⁴、-CH=N-R⁴或-CH₂-R⁵,其中R⁴为酰基、酰胺基、杂环氨基、杂环(较低)烷基或芳基(较低)烷氧基,R⁵为酰氧基或杂环(较低)烷氧基,X为-O-或-CH₂-,或其药学上可接受的盐,以及其制备方法和包含它们作为活性成分的药物组合物。
-
Salmi, Chemische Berichte, 1938, vol. 71, p. 1803,1807作者:SalmiDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
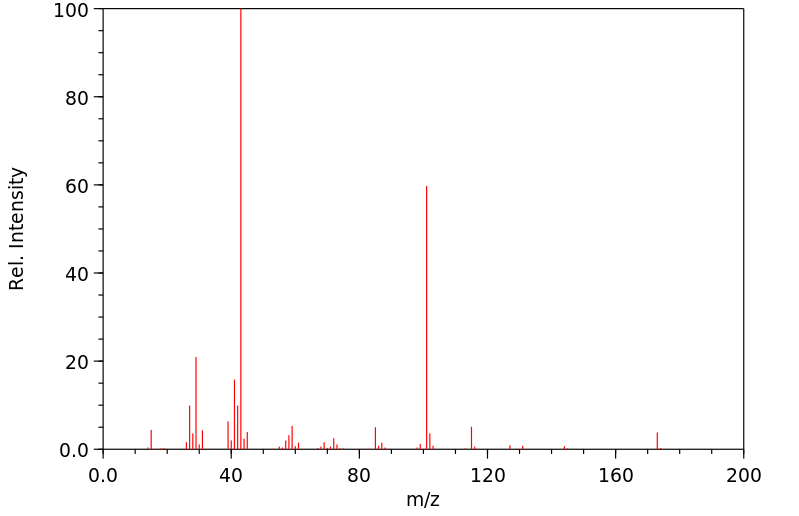
质谱MS
-
碳谱13CNMR
-
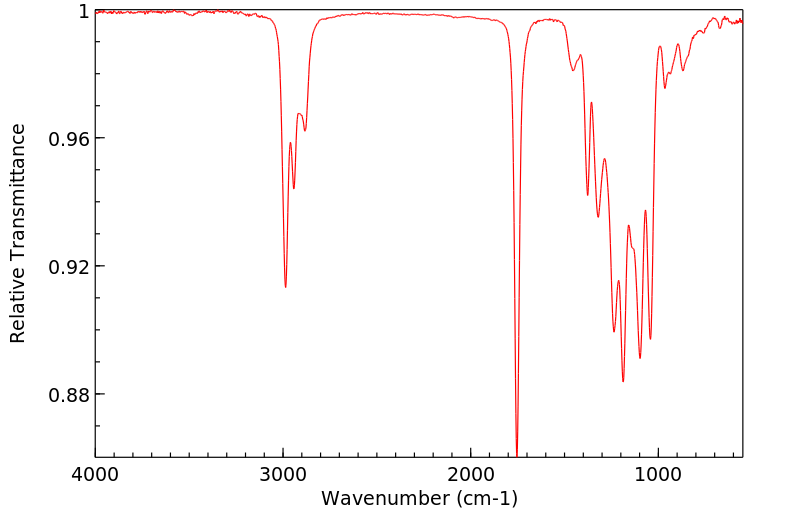
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯