N-Phenyl-2-methoxybenzylideneamine | 104669-25-2
中文名称
——
中文别名
——
英文名称
N-Phenyl-2-methoxybenzylideneamine
英文别名
N-(2-methoxybenzylidene)aniline;(E)-1-(2-methoxyphenyl)-N-phenylmethanimine;(E)-N-(2-methoxybenzylidene)aniline;N-[(2-methoxy)benzylidene]aniline;N-phenyl-2-methoxybenzylidenimine;N-2-methoxybenzylideneaniline
CAS
104669-25-2
化学式
C14H13NO
mdl
MFCD00025757
分子量
211.263
InChiKey
HBBUHFXBDZPAMN-RVDMUPIBSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.69
-
重原子数:16.0
-
可旋转键数:3.0
-
环数:2.0
-
sp3杂化的碳原子比例:0.071
-
拓扑面积:21.59
-
氢给体数:0.0
-
氢受体数:2.0
反应信息
-
作为反应物:描述:N-Phenyl-2-methoxybenzylideneamine 在 2,3-二氯-5,6-二氰基-1,4-苯醌 作用下, 以 乙醚 、 苯 为溶剂, 反应 4.0h, 生成 N-(2-methoxy-α-phenylbenzylidene)aniline参考文献:名称:A route to sterically crowded benzophenone N-aryl imines摘要:DOI:10.1021/jo00271a047
-
作为产物:参考文献:名称:芳基硼酸与芳烃通过Rh催化的直接CH芳基化氧化偶联。摘要:[RhCl(C2H4)2] 2 / P [p-(CF3)C6H4] 3催化剂体系实现了三种不同芳烃和噻吩衍生物与各种芳基硼酸的氧化偶联。使用可商购的2,2,6,6-四甲基哌啶-N-氧基自由基(TEMPO)作为化学计量的氧化剂。2-吡啶基和亚胺官能团用作邻位导向基团,以通过Rh络合物介导直接CH芳基化。对于偶联反应,获得了中等至优异的产率。DOI:10.1021/ol702659a
文献信息
-
Rhodium-Catalyzed Synthesis of Imines and Esters from Benzyl Alcohols and Nitroarenes: Change in Catalyst Reactivity Depending on the Presence or Absence of the Phosphine Ligand作者:Taemoon Song、Ji Eun Park、Young Keun ChungDOI:10.1021/acs.joc.8b00197日期:2018.4.6catalyzes the reductive N-alkylation of aryl nitro compounds with alcohols by a borrowing-hydrogen strategy to afford the corresponding imine products in good to excellent yields. In the absence of xantphos, the [Rh(COD)Cl]2/Cs2CO3 catalytic system behaves as an effective catalyst for the dehydrogenative coupling of alcohols to esters, with nitrobenzene as a hydrogen acceptor. The reactivity of the
-
Imino-Diels–Alder and imino-aldol reactions catalyzed by samarium diiodide作者:Jacqueline Collin、Nada Jaber、Marie Isabelle LannouDOI:10.1016/s0040-4039(01)01581-7日期:2001.10In the presence of a catalytic amount of samarium diiodide in methylene chloride, aromatic imines react with Danishefsky diene to form tetrahydropyridine-4-ones in high yields. Under the same conditions, various imino-aldol reactions afford β-amino esters or β-amino ketones.
-
STABILIZATION OF ACTIVE METAL CATALYSTS AT METAL-ORGANIC FRAMEWORK NODES FOR HIGHLY EFFICIENT ORGANIC TRANSFORMATIONS申请人:The University of Chicago公开号:US20180361370A1公开(公告)日:2018-12-20Metal-organic framework (MOFs) compositions based on post¬synthetic metalation of secondary building unit (SBU) terminal or bridging OH or OH 2 groups with metal precursors or other post-synthetic manipulations are described. The MOFs provide a versatile family of recyclable and reusable single-site solid catalysts for catalyzing a variety of asymmetric organic transformations, including the regioselective boryiation and siiylation of benzyiic C—H bonds, the hydrogenation of aikenes, imines, carbonyls, nitroarenes, and heterocycles, hydroboration, hydrophosphination, and cyclization reactions. The solid catalysts can also be integrated into a flow reactor or a supercritical fluid reactor.
-
Insight into the Modes of Activation of Pyridinium and Bipyridinium Salts in Non‐Covalent Organocatalysis作者:Robin Weiss、Tamara Golisano、Patrick Pale、Victor MamaneDOI:10.1002/adsc.202100865日期:2021.10.19A series of pyridinium and bipyridinium salts were prepared and their catalytic properties were evaluated in the aza-Diels-Alder reaction between imines and Danishefsky diene. Depending on the substituents of the pyridinium/bipyridinium rings and on the nature of the counterion, two mechanisms of activation were demonstrated. In case of non-substituted rings, the substrate is activated through charge制备了一系列吡啶鎓盐和联吡啶鎓盐,并在亚胺和丹麦谢夫斯基二烯之间的氮杂-狄尔斯-阿尔德反应中评估了它们的催化性能。根据吡啶鎓/联吡啶鎓环的取代基和抗衡离子的性质,证明了两种活化机制。在未取代环的情况下,底物通过涉及亚胺 C 侧芳环的电荷转移而被激活。当在催化剂上引入卤素原子时,活化模式切换到涉及亚胺氮孤对的卤素键。此外,排除了基于氢键和自由基阳离子的替代激活模式。这项工作使我们能够开发出两类催化剂,它们的潜力在各种亚胺与丹麦谢夫斯基二烯的环加成反应中得到了证明。第一个家族由简单的甲基吡啶三氟甲磺酸盐和二辛基联吡啶三氟甲磺酸盐组成。前者仅对带有 a 的亚胺有效发现 C 侧的对甲氧基苯基和后者对于在亚胺的 N 侧和 C 侧带有不同取代基的亚胺是有效的。第二类是基于卤化吡啶鎓盐,它被证明对几乎所有考虑的亚胺都有活性。
-
Tris(2,4,6-trifluorophenyl)borane: An Efficient Hydroboration Catalyst作者:James R. Lawson、Lewis C. Wilkins、Rebecca L. MelenDOI:10.1002/chem.201703109日期:2017.8.16The metal‐free catalyst tris(2,4,6‐trifluorophenyl)borane has demonstrated its extensive applications in the 1,2‐hydroboration of numerous unsaturated reagents, namely alkynes, aldehydes and imines, consisting of a wide array of electron‐withdrawing and donating functionalities. A range of over 50 borylated products are reported, with many reactions proceeding with low catalyst loading under ambient
表征谱图
-
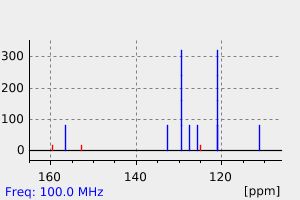
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯