2-phenyl-2H-phenanthro[9,10-d][1,2,3]triazole | 973-14-8
中文名称
——
中文别名
——
英文名称
2-phenyl-2H-phenanthro[9,10-d][1,2,3]triazole
英文别名
2-Phenyl-2H-phenanthro(9,10-d)triazole;2-phenylphenanthro[9,10-d]triazole
CAS
973-14-8
化学式
C20H13N3
mdl
——
分子量
295.343
InChiKey
YEYUESDYKNXPEY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:23
-
可旋转键数:1
-
环数:5.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:30.7
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:2-phenyl-2H-phenanthro[9,10-d][1,2,3]triazole 、 硫酸二甲酯 在 sodium perchlorate 作用下, 生成 1-Methyl-2-phenyl-2H-phenanthro[9,10-d][1,2,3]triazol-1-ium; perchlorate参考文献:名称:New phenanthrene systems: spiro-fused tricyclic phenanthro-1,2,4-triazolo-[4,3-d]-1,2,4-triazines and -[3,4-d]-1,2,5-oxadiazines: ring expansions of N-methylphenanthroazoliums摘要:介绍了取代菲罗[9,10-e]-1,2,4-三嗪和菲罗[9,10-c]-1,2,5-恶二嗪的新路线。 这些化合物与 C-苯基-N-对硝基苯腈亚胺[IUPAC 名称:2-苄叉-1-(对硝基苯基)肼-2-鎓-1-ide]发生环加成反应,生成了新的取代的螺三环菲并-1,2,4-三唑并[4,3-d][1,2,4]三嗪和菲并-1,2,4-三唑并[3,4-d][1,2,5]恶二嗪。报告了 7,10-二苯基-5-(4′-硝基苯基)-9,10-二氢-5H-菲罗[9,10-e][1,2,4]三唑并[4,3-d][1,2,4]三嗪 5a 的 X 射线晶体结构。DOI:10.1039/a605770e
-
作为产物:描述:9,10-Bis(phenylazo)phenanthrene 在 三氯化磷 作用下, 反应 1.0h, 以93%的产率得到2-phenyl-2H-phenanthro[9,10-d][1,2,3]triazole参考文献:名称:Tricyclic phenanthrene systems: substituted phenanthro[9,10-e]-1,2,3-triazines and fused phenanthro-azolo-1,2,3-triazoles from cycloaddition—rearrangement sequences of 9,10-bisarylazophenanthrenes with 2π-dipolarophiles. Azolium 1,3-dipoles摘要:A range of new fused ring systems based on phenanthrene were obtained from cycloaddition-rearrangement reactions of 9, 10-bisarylazophenanthrenes with alkyne and alkene dipolarophilles. These new rings include substituted phenanthro[9,10-e]-1,2,3-triazines, the tricyclic systems, substituted 3a,6a-(biphen-2,2'-yl)hexahydropyrrolo[2, 3-d]-1,2,3-triazoles and substituted 3a,6a-(biphen-2,2'-yl)hexahydroimidazo[4,5-d]-1,2,3-triazoles. X-Ray crystal structures are reported on 2-(p-bromophenyl)-4 -methoxycarbonyl-4-(p-bromophenyliminomethoxalyl)-3,4-dihydrophenanthro[9,10-e]-1,2,3-triazin-2-ium-3-ide 6b, and 2,4-diphenyl-3a,6a-(biphen-2,2'-yl)-5,6-hexahydropyrrolo[2,3-d]-1,2, 3-triazol-2-ium-1-ide 11a.DOI:10.1039/p19960001623
文献信息
-
Competitive reactivity of the aryl isothiocyanate dipolarophile at NC versus CS with nucleophilic 1,3-dipoles: a combined experimental and theoretical study. The reactions of substituted 1,2,3-triazolium-1-aminide 1,3-dipoles with aryl isothiocyanates: new tricyclic thiazolo[4,5-d][1,2,3]triazoles作者:Richard N. Butler、Denise C. Grogan、Peter D. McDonald、Luke A. BurkeDOI:10.1039/a705233b日期:——Substituted 1,2,3-triazolium-1-aminide 1,3-dipoles react with aryl isothiocyanates at both the NC and CS sites to give mixtures of substituted imidazolo[4,5-d][1,2,3]triazoles and new thiazolo[4,5-d][1,2,3]triazoles including tricyclic derivatives with the C-3a and C-6a bridgeheads linked via (CH2)4 and phenanthro groups. The product distribution is controlled by the para-substituent of the aryl isothiocyanate. Theoretical calculations, 3-21G* and 6-31G*, suggest that linear triple bonded canonical forms of the aryl isothiocyanate system play a key role in the ambident reactivity of these systems.
-
OLED WITH HYBRID EMISSIVE LAYER申请人:The University of Southern California公开号:US20190237694A1公开(公告)日:2019-08-01A hybrid emissive layer and OLED incorporating the same are provided. The hybrid emissive layer includes a first material having a triplet state energy level T1 H and a singlet state energy level S1 H , a second material having a triplet state energy level T1 F and a singlet state energy level S1 F ; and a third material having a triplet state energy level T1 P and a single state energy level S1 P , where T1 F ≥T1 H ; S1 F ≤S1 H ; and T1 P查看更多
表征谱图
-
氢谱1HNMR
-
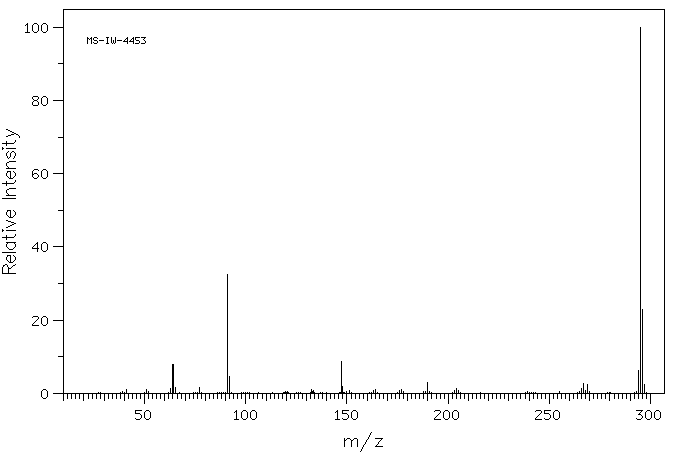
质谱MS
-
碳谱13CNMR
-
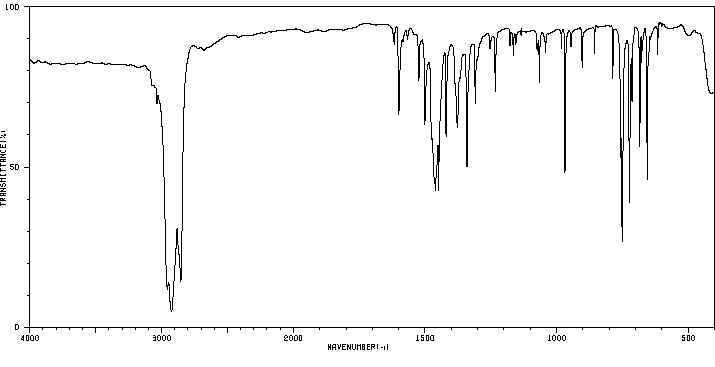
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇 (6,6)-苯基-C61己酸甲酯 高雌二醇 马兜铃酸钠 马兜铃酸盐 马兜铃酸C 马兜铃酸B 马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII) 马兜铃酸 Ia 马兜铃酸 IVa 马兜铃酸 颜料黑32 颜料红179 颜料红178 颜料红149 颜料红123 顺式-菲-1,2-二醇-3,4-环氧化物 顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物 雷公藤酚A 镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯) 钩大青酮 钩大青酮 钙(2+)12-羟基十八烷酸酯 酒石酸布托诺啡 那布扶林 还原红32 足球烯 贝那他汀B 贝母兰素 萘并[2,3-b]荧蒽 萘并[2,1-e][1]苯并二硫杂环戊烷 萘并[2,1-C:7,8-C']二菲 萘并[1,2-e][2]苯并呋喃-1,3-二酮 萘并[1,2-b]屈 萘并[1,2-a]蒽 萘并[1,2-B]菲-6-醇 萘二(六氯环戊二烯)加合物 萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基- 菲醌单缩氨基硫脲 菲醌 菲并[9,10]呋喃 菲并[9,10-e]醋菲烯 菲并[4,5-bcd]噻吩 菲并[4,5-bcd]呋喃-3-醇 菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基- 菲并[3,2-b]噻吩 菲并[2,1-d]噻唑 菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮 菲并(3,4-b)噻吩 菲并(1,2-b)噻吩 -