溴化氰 | 506-68-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:50-53 °C (lit.)
-
沸点:61-62 °C (lit.)
-
密度:1.443 g/mL at 25 °C
-
蒸气密度:3.65 (vs air)
-
闪点:61.4°C
-
溶解度:易溶于氯仿、二氯甲烷、乙醇、乙醚、苯和乙腈。
-
暴露限值:No exposure limit is set. However, on the basis of the exposure limits of related compounds a ceiling limit of 0.5 ppm (2 mg/m3) is recommended.
-
物理描述:Cyanogen bromide is a colorless to white crystalline solid with a penetrating odor. It is slightly soluble in water. It is gradually decomposed by water and very rapidly by acids to give off hydrogen bromide, a flammable and poisonous gas. Contamination with many materials can cause rapid decomposition of the material. It is toxic by inhalation of its vapors or by the hydrogen cyanide from decomposition or by ingestion. Toxic oxides of nitrogen are produced in fire involving this material. It is used in gold extraction, to make other chemicals, and as a fumigant.
-
颜色/状态:White hygroscopic needles
-
气味:Penetrating
-
味道:Bitter
-
蒸汽密度:3.62 (EPA, 1998) (Relative to Air)
-
蒸汽压力:122 mm Hg at 25 °C
-
稳定性/保质期:
-
自燃温度:Not Applicable. Not flammable. (USCG, 1999)
-
分解:... If involved in a fire decomposes to produce toxic gases.
-
腐蚀性:Corrodes most metals
-
汽化热:33.82 kJ/mol
-
保留指数:482;482
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:3
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:23.8
-
氢给体数:0
-
氢受体数:1
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T+,N
-
安全说明:S16,S26,S28,S36/37/39,S45,S53,S60,S61
-
危险类别码:R26/27/28,R34,R50/53,R40
-
WGK Germany:3
-
海关编码:2853009021
-
危险品运输编号:UN 3390 6.1/PG 1
-
危险类别:6.1
-
RTECS号:GT2100000
-
包装等级:I
-
储存条件:储存注意事项: - 储存在阴凉、通风的库房中。 - 远离火源和热源。 - 包装需密封,避免与空气接触。 - 应与氧化剂、碱类及食用化学品分开存放,切忌混储。 - 不宜大量储存或长期存放。 - 储存时应准备合适的材料以处理泄漏物。
制备方法与用途
性质
溴化氰是一种无色固体,剧毒且容易吸潮。其熔点为50-53℃,沸点为61-62℃,易升华。
溴化氰活化
溴化氰活化法是常用的活化方法之一,主要通过生成亚胺碳酸活性基团与伯氨基(NH₂)反应,生成异脲衍生物。这种方法在温和条件下可以与配体结合,且结合量较大。然而,使用溴化氰活化的基质与配体偶联后会生成带有正电荷的异脲衍生物,导致阴离子交换作用和非特异性吸附,从而影响亲和层析的分辨率。此外,由于其不稳定性,在小分子配体的情况下可能会出现脱落现象。另外,溴化氰本身剧毒且易挥发,操作不便。
目前市场上主要使用溴化氰活化和环氧活化方法。溴化氰活化的优点是载量高、反应速度快,但连接处带有正电荷,可能引起阴离子交换作用,并且结合不够牢固。而环氧活化则结合更稳定,非特异性吸附更低,但载量低,反应速度极慢。
最近市场上出现了CDI(对甲苯磺酰氯)活化的偶联试剂盒产品,这被认为是溴化氰活化的升级版。CDI活化的产品具有更高的载量、更快的反应速度以及更强的结合稳定性,且没有非特异性吸附。这些试剂盒配备了合适的缓冲液和耗材,使得操作变得更为简便快捷。
溴化氰毒性
溴化氰与水或水蒸气接触时会释放剧毒、易燃及腐蚀性的溴化氢和氰化氢气体。不纯物质的存在可能会导致其快速分解并引起爆炸。吸入溴化氰会导致头痛、头晕、恶心、呕吐等症状,严重时可致肺水肿甚至死亡。对眼睛和皮肤有强烈刺激性。口服后会引起口腔和胃的灼伤,可能导致死亡。
燃烧(分解)产物包括氰化氢和溴化氢。与氢氧化钠反应生成氰酸钠,与氨及胺作用生成氨基氰,与醇作用生成三聚氰酸酯。
溴化氰在受热或遇水时会释放剧毒气体,并且遇酸容易引起爆炸。本品不燃,在火场中存在溴化氰的情况下可以用雾状水进行灭火,但需避免水直接接触溴化氰。禁止使用酸碱灭火剂。含氰化物的废水必须经过处理后再排放,并应与酸性废水分开储存以防止氰化氢气体逸出。
用途
- 用于支持物琼脂糖凝胶(Sepharose 4B)结合,通过亲和层析法实现纯化或活化。例如:疫苗;单克隆抗体。
- 吡啶环的开环反应鉴别,如尼可刹米的鉴别。
- 肉与肉制品中的维生素PP含量测定。
- 在蛋白质序列测定中断裂肽键(甲硫氨酸侧链的硫醚基),如将免疫球蛋白分解为片段,例如Fc段、Fab段。
类别
有毒物品
毒性分级
剧毒
急性毒性
吸入-小鼠 LCL0: 0.5 克/立方米/10分
可燃性危险特性
不燃;遇水、潮气和酸分解产生有毒氢化氢气体及易燃气体溴化氢。
储运特性
库房通风低温干燥,与酸类分开储存运输。
灭火剂
雾状水。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 氰溴化物-13C Cyanogen bromide-13C 70610-98-9 CBrN 106.911
反应信息
-
作为反应物:参考文献:名称:WO2006/44405摘要:公开号:
-
作为产物:参考文献:名称:Langlois, Annales de Chimie (Cachan, France), 1861, vol. <3>61, p. 482摘要:DOI:
-
作为试剂:参考文献:名称:New synthetic protocol for stereoselective synthesis of diethyl 1,2-dicyano-3-alkyl-(aryl)cyclopropane-1,2-dicarboxylate摘要:开发了一种新的、快速且直接的一锅反应方法,用于芳香和脂肪醛以及二醛与氰乙酸乙酯和溴化氰的反应,以高产率和短反应时间(约5秒)选择性地合成二乙基1,2-二氰基-3-烷基/芳基环丙烷-1,2-二羧酸酯。通过红外光谱(IR)、氢核磁共振(1H NMR)和碳核磁共振(13C NMR)对其结构进行了表征。讨论了反应机理。DOI:10.1007/s13738-015-0590-3
文献信息
-
[EN] IMIDAZOLE DERIVATIVES USEFUL AS INHIBITORS OF FAAH<br/>[FR] DÉRIVÉS IMIDAZOLE UTILES COMME INHIBITEURS DE LA FAAH申请人:MERCK & CO INC公开号:WO2009152025A1公开(公告)日:2009-12-17The present invention is directed to certain imidazole derivatives which are useful as inhibitors of Fatty Acid Amide Hydrolase (FAAH). The invention is also concerned with pharmaceutical formulations comprising these compounds as active ingredients and the use of the compounds and their formulations in the treatment of certain disorders, including osteoarthritis, rheumatoid arthritis, diabetic neuropathy, postherpetic neuralgia, skeletomuscular pain, and fibromyalgia, as well as acute pain, migraine, sleep disorder, Alzeimer Disease, and Parkinson's Disease.
-
[EN] THIOPHENE DERIVATIVES FOR THE TREATMENT OF DISORDERS CAUSED BY IGE<br/>[FR] DÉRIVÉS DE THIOPHÈNE POUR LE TRAITEMENT DE TROUBLES PROVOQUÉS PAR IGE申请人:UCB BIOPHARMA SRL公开号:WO2019243550A1公开(公告)日:2019-12-26Thiophene derivatives of formula (I) and a pharmaceutically acceptable salt thereof are provided. These compounds have utility for the treatment or prevention of disorders caused by IgE, such as allergy, type 1 hypersensitivity or familiar sinus inflammation.
-
[EN] CATHEPSIN CYSTEINE PROTEASE INHIBITORS<br/>[FR] INHIBITEURS DE PROTÉASES À CYSTÉINE DE TYPE CATHEPSINES申请人:MERCK SHARP & DOHME公开号:WO2015054038A1公开(公告)日:2015-04-16This invention relates to a novel class of compounds which are cysteine protease inhibitors, including but not limited to, inhibitors of cathepsins K, L, S and B. These compounds are useful for treating diseases in which inhibition of bone resorption is indicated, such as osteoporosis.
-
Insecticidal and acaricidal diarylpyrrolecarbonitrile and申请人:American Cyanamid Company公开号:US05180734A1公开(公告)日:1993-01-19This invention relates to new diarylpyrrolecarbonitrile and new diarylnitropyrrole compounds. It also relates to the use of said compounds as insecticidal and acaricidal agents and to a method of protecting plants, particularly crop plants, from attack by insects and acarina by application of a new diarylpyrrolecarbonitrile or diarylnitropyrrole to said plants or to the locus in which they are growing.
-
[EN] COMPOUNDS AND THEIR USE AS BACE INHIBITORS<br/>[FR] COMPOSÉS ET LEUR UTILISATION EN TANT QU'INHIBITEURS DE BACE申请人:ASTRAZENECA AB公开号:WO2016055858A1公开(公告)日:2016-04-14The present application relates to compounds of formula (I), (la), or (lb) and their pharmaceutical compositions/preparations. This application further relates to methods of treating or preventing Αβ-related pathologies such as Down's syndrome, β- amyloid angiopathy such as but not limited to cerebral amyloid angiopathy or hereditary cerebral hemorrhage, disorders associated with cognitive impairment such as but not limited to MCI ("mild cognitive impairment"), Alzheimer's disease, memory loss, attention deficit symptoms associated with Alzheimer's disease, neurodegeneration associated with diseases such as Alzheimer's disease or dementia, including dementia of mixed vascular and degenerative origin, pre-senile dementia, senile dementia and dementia associated with Parkinson's disease.本申请涉及式(I)、(Ia)或(Ib)的化合物及其药物组合物/制剂。本申请进一步涉及治疗或预防与Αβ相关的病理学,如唐氏综合症,β-淀粉样蛋白血管病,如但不限于脑淀粉样蛋白血管病或遗传性脑出血,与认知损害相关的疾病,如但不限于MCI(“轻度认知损害”),阿尔茨海默病,记忆丧失,与阿尔茨海默病相关的注意力缺陷症状,与疾病如阿尔茨海默病或痴呆症相关的神经退行性疾病,包括混合性血管性和退行性起源的痴呆,早老性痴呆,老年性痴呆和与帕金森病相关的痴呆的方法。
表征谱图
-
氢谱1HNMR
-
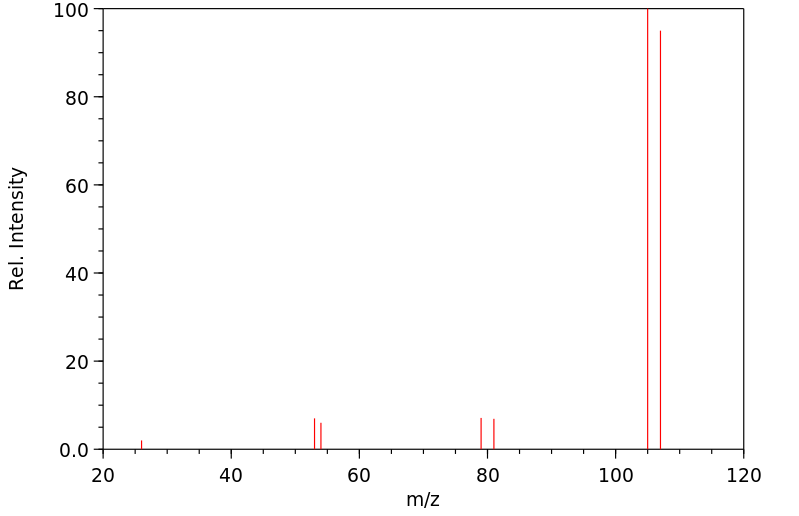
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息