bis-(2,4-dimethyl-phenyl)methane | 32588-46-8
中文名称
——
中文别名
——
英文名称
bis-(2,4-dimethyl-phenyl)methane
英文别名
Bis-(2,4-dimethyl-phenyl)-methane;1-[(2,4-dimethylphenyl)methyl]-2,4-dimethylbenzene
CAS
32588-46-8
化学式
C17H20
mdl
——
分子量
224.346
InChiKey
YKFHEULXQNPVRH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:127 °C
-
沸点:140-142 °C(Press: 3 Torr)
-
密度:0.958±0.06 g/cm3(Predicted)
-
保留指数:1822;1823
计算性质
-
辛醇/水分配系数(LogP):5.3
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.29
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,2,4,4-四甲基苯甲酮 2,2',4,4'-tetramethylbenzophenone 3478-88-4 C17H18O 238.329
反应信息
-
作为反应物:描述:参考文献:名称:Production of aromatic compounds摘要:公开号:US02819322A1
-
作为产物:描述:2,2-bis(2,4-dimethylphenyl)-1,3-dithiane 在 N,N,N',N'-tetramethyl-7,8-dihydro-6H-dipyrido[1,2-a;2',1'-c][1,4]diazepine-2,12-diamine 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 24.0h, 以35.1 mg的产率得到bis-(2,4-dimethyl-phenyl)methane参考文献:名称:超电子给体介导的还原性脱硫反应†摘要:描述了通过吡啶衍生的电子供体对硫缩醛和硫醚的脱硫。这种方法可以高效地获得高产率的还原产物,并且不需要使用过渡金属,元素碱金属或氢原子供体。DOI:10.1039/c9cc06775b
文献信息
-
Decarbonylative Diarylation of α-Methoxyacetic Acid Yielding Diarylmethanes Mediated by Lewis Acid and Trifluoroacetic Anhydride作者:Takashi Jobashi、Tetsuo Hino、Katsuya Maeyama、Hiroyuki Ozaki、Kenji Ogino、Noriyuki YonezawaDOI:10.1246/cl.2005.860日期:2005.6Reaction of α-methoxyacetic acid with aromatic compounds in the presence of trifluoroacetic anhydride and Lewis acid has been found to give diarylmethanes. Some of the intermediates in this transformation have been identified via direct observation by 1H and 13C NMR spectroscopy. The reaction route has been clarified as follows: a mixed acid anhydride is formed from α-methoxyacetic acid and trifluoroacetic anhydride, which gives a hemiacylal type intermediate via decarbonylation followed by successive double electrophilic aromatic substitutions yielding diarylmethanes.
-
Structural requirements for decarbonylative α,α-diarylation reaction of 2-methoxyalkanoic acids in phosphorus pentoxide-methanesulfonic acid mixture yielding 1,1-diarylalkane homologs作者:Noriyuki Yonezawa、Tetsuo Hino、Yoshimi Tokita、Kazuhisa Matsuda、Tomiki IkedaDOI:10.1016/s0040-4020(97)00996-4日期:1997.102-Methoxyalkanoic acids were found to undergo consecutive decarbonylative α,α-diarylation in P2O5-MsOH instead of Friedel-Crafts type arylation on the carbonyl carbon. The influence of the substituents of the arenes and the carboxylic acids in this reaction was elucidated based on the reaction yields. The reaction behavior was found to be primarily governed by the electron-withdrawing/releasing property
-
Functional Ionic Liquids as Efficient and Recyclable Catalysts for the Methylation of Formaldehyde with Aromatics作者:Heyuan Song、Fuxiang Jin、Ronghua Jin、Meirong Kang、Zhen Li、Jing ChenDOI:10.1007/s10562-016-1750-5日期:2016.7Methylation of formaldehyde with various aromatics under functional ionic liquids catalysis has been developed. Among the ionic liquids investigated, triphenyl-(4-sulfobutyl)-phosphonium triflate ([TTPBs][CF3SO3]) showed high activity and afforded excellent yields of diarylmethane derivatives. A mechanism for the catalytic performance of [TTPBs][CF3SO3] is proposed. Besides, the catalyst can simply
-
Shapiro, I. I.; Ranneva, Yu. I.; Shatenshtein, A. I., Journal of general chemistry of the USSR, 1981, vol. 51, p. 2191 - 2195作者:Shapiro, I. I.、Ranneva, Yu. I.、Shatenshtein, A. I.DOI:——日期:——
-
Identification of alkylarene chloromethylation products using gas-chromatographic retention indices作者:I. G. Zenkevich、A. A. MakarovDOI:10.1134/s1070363207040196日期:2007.4Gas-chromatographic retention indices on standard nonpolar polydimethylsiloxane stationary phases allow identification of products formed by known organic reactions even without using mass-spectrometric data. The efficiency of this approach was demonstrated by the example of identification of previously uncharacterized chloromethyl derivatives of alkylarenes, including structural isomers of compounds containing several chloromethyl groups, directly in reaction mixtures. Chromatographic analysis of such reaction mixtures allows identification of positional isomers of the starting alkylarenes even when they are present simultaneously. The retention indices were determined for the first time for more than 50 alkyl-(chloromethyl)arenes, by-products of chloromethylation, and chloromethyl derivatives of the simplest alkyl phenyl ketones.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
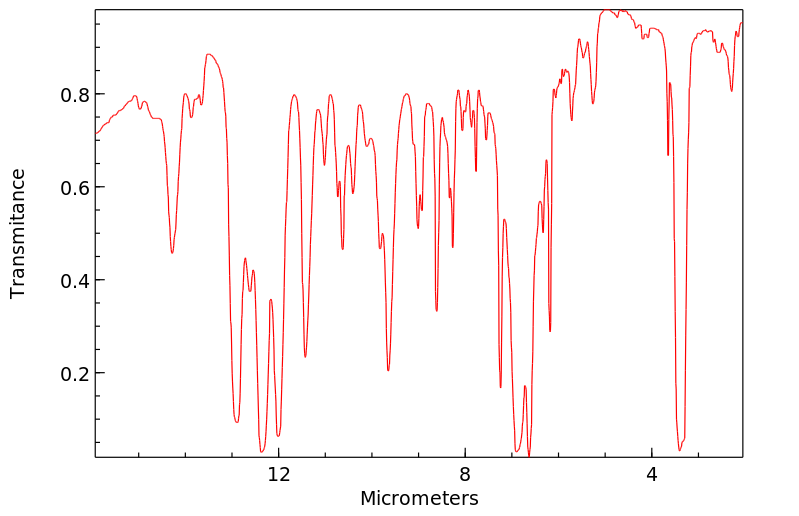
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫