biscyclohexa-1,4-diselena-2,5-cyclohexadiene | 35831-82-4
中文名称
——
中文别名
——
英文名称
biscyclohexa-1,4-diselena-2,5-cyclohexadiene
英文别名
bis(cyclohexeno)-1,4-diselenine;bis(cyclohexeno)-1,4-diselenin;1,2,3,4,6,7,8,9-octahydro-selenanthrene;Bis-cyclohexeno-1,4-diselenin;1,2,3,4,6,7,8,9-Octahydroselenanthrene
CAS
35831-82-4
化学式
C12H16Se2
mdl
——
分子量
318.179
InChiKey
AABWQLCKLNUTEB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:419.7±40.0 °C(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.98
-
重原子数:14
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Dinuclear diselenolenes derived from cycloalkeno-1,2,3-selenadiazoles and tetrakis(triphenylphosphine)palladium摘要:在甲苯中,[Pd(PPh3)4] 与环烯-1,2,3-硒二唑或双(环烯)-1,4-二硒化物在回流条件下发生反应,产生了双核二硒化物 [Pd2{SeC(R1)C(R2)Se}2(PPh3)2] [R1,R2 = (CH2)n;n = 4、5、6],这些化合物已通过显微分析、多核核磁共振、红外光谱和质谱进行了表征;n = 6 的化合物的分子结构已通过 X 射线晶体学确定。DOI:10.1039/a807666i
-
作为产物:描述:4,5,6,7-Tetrahydrocyclohexa-1,2,3-selenadiazol 以 neat (no solvent) 为溶剂, 以60%的产率得到biscyclohexa-1,4-diselena-2,5-cyclohexadiene参考文献:名称:微波合成双(环烯基)-1,4-二硒精:CdSe QDs的硒的新来源†摘要:本文介绍了通过微波辐射(MW)方法由相应的环烯基1,2,3-硒代二唑形成一系列的双(环烯基)-1,4-二硒精。1,2,3-硒代二唑的双自由基二聚反应是通过一种新的合成策略在无溶剂条件下使用100瓦特微波辐照约20分钟进行的。当前的合成为制备各种1,4-二硒精提供了可行的方法。如此制备的烷基硒化物通过各种光谱学工具表征。紫外线可见,FTIR,1 H,13 C,DEPT和77Se NMR光谱,ESI-MS和TGA分析。另外,双(环庚烯)-1,4-二硒精已被有效地用作合成CdSe量子点(QDs)的新型硒前体。这种富含硒的前体也可能用于制备其他金属硒化物。DOI:10.1039/c7nj00793k
文献信息
-
Synthesis and Properties of Palladium Diselenolenes: X-ray Crystal Structures of [Pd{SeC(R<sup>1</sup>)C(R<sup>2</sup>)Se}(PBu<sub>3</sub>)<sub>2</sub>] [R<sup>1</sup>, R<sup>2</sup> = (CH<sub>2</sub>)<i><sub>n</sub></i>, <i>n</i> = 4, 5, 6]作者:Susan Ford、Christopher P. Morley、Massimo Di VairaDOI:10.1021/ic040069y日期:2004.11.1The reaction between [Pd(2)(dba)(3)] (dba = dibenzylideneacetone), tributylphosphine, and a bis(cycloalkeno)-1,4-diselenin leads to either a mononuclear diselenolene [Pd[SeC(R(1))=C(R(2))Se](PBu(3))(2)] or a dinuclear diselenolene [Pd(2)[SeC(R(1))=C(R(2))Se](2)(PBu(3))(2)] [R(1), R(2) = (CH(2))(n), n = 4, 5, 6] depending on the stoichiometry employed. Treatment of the dinuclear diselenolenes with 1[Pd(2)(dba)(3)](dba =二苄叉基丙酮),三丁基膦和双(环烯基)-1,4-二硒烯之间的反应会导致单核二硒烯[Pd [SeC(R(1)) )= C(R(2))Se](PBu(3))(2)]或双核二硒烯[Pd(2)[SeC(R(1))= C(R(2))Se](2 )(PBu(3))(2)] [R(1),R(2)=(CH(2))(n),n = 4,5,6],取决于所用的化学计量。用1,2-双(二苯基膦基)乙烷(dppe)处理双核二硒烯为单核物质[Pd [SeC(R(1))= C(R(2))Se](dppe)提供了高产途径)]。所有新化合物均已通过标准光谱学和分析技术进行了表征,尤其是通过多核NMR光谱学进行了表征;每个单核三丁基膦配合物的结构已经通过X射线晶体学测定。
-
The reaction of 1,2,3-selenadiazole with olefins作者:Yutaka Nishiyama*、Yasunobu Hada、Kuniko Iwase、Noboru Sonoda*DOI:10.1016/s0022-328x(00)00484-8日期:2000.10treated with an excess amount of olefins at 130°C, the addition of a vinyl radical, which was generated in situ by the denitrogenation of 1,2,3-selenadiazoles, to a carbon–carbon double bond followed by intramolecular cyclization proceeded efficiently giving the corresponding dihydroselenophenenes in moderate to good yields along with the formation of the corresponding 1,4-diselenins and selenophenes
-
Tributylstannyl Radical-Catalyzed Reaction of 1,2,3-Selenadiazoles with Olefins or Dienes作者:Yutaka Nishiyama、Yasunobu Hada、Masahiro Anjiki、Kazuya Miyake、Sakiko Hanita、Noboru SonodaDOI:10.1021/jo010893t日期:2002.3.1It was found that the reaction of 1,2,3-selenadiazoles derived from cyclic ketones with olefins or dienes was markedly promoted by a catalytic amount of tributylstannyl radical, which was generated in situ from tributylstannyl hydride or allyltributylstannane and AIBN, to give the corresponding dihydroselenophenes in moderate to good yields. In contrast, when 1,2,3-selenadiazoles prepared from linear
-
Catalytic use of organostannyl radical: The reaction of 1,2,3-selenadiazole with olefins in the presence of a catalytic amount of tributyltin hydride作者:Yutaka Nishiyama、Yasunobu Hada、Masahiro Anjiki、Sakiko Hanita、Noboru SonodaDOI:10.1016/s0040-4039(99)01286-1日期:1999.8When 1,2,3-selenadiazoles were treated with an excess amount of olefins in the presence of a catalytic amount of Bu3SnH and AIBN, the addition of a vinyl radical, which was generated in situ by the denitrogenation of 1,2,3-selenadiazoles, to the carbon-carbon double bond followed by intramolecular cyclization proceeded efficiently to afford the corresponding dihydroselenophenes in moderate to good
-
Synthesis of mono- and di-nuclear palladium diselenolenes from bis(cycloalkeno)-1,4-diselenins: X-ray crystal structure of [Pd(C7H10Se2)(PBu3)2]作者:Susan Ford、Christopher P. Morley、Massimo Di VairaDOI:10.1039/a904853g日期:——The reaction between [Pd2(dba)3] (dba=dibenzylideneacetone), tributylphosphine, and a bis(cycloalkeno)-1,4-diseleninleads to either a mononuclear [PdSeC(R1)2 C(R2)Se}(PBu3)2], or a dinuclear [Pd2SeC(R1)2 C(R2)Se}2(PBu3)2] diselenolene (R1–R2=(CH2) n ; n =4, 5,6) depending on the stoichiometry employed; the structure of the monomeric product with n =5 has been determined by X-ray crystallography.
表征谱图
-
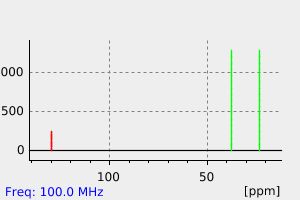
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
()-2-(5-甲基-2-氧代苯并呋喃-3(2)-亚乙基)乙酸乙酯
(双(2,2,2-三氯乙基))
(乙基N-(1H-吲唑-3-基羰基)ethanehydrazonoate)
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(S)-(-)-2-(α-(叔丁基)甲胺)-1H-苯并咪唑
(S)-(-)-2-(α-甲基甲胺)-1H-苯并咪唑
(S)-氨氯地平-d4
(S)-8-氟苯并二氢吡喃-4-胺
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(S)-4-氯-1,2-环氧丁烷
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(S)-2-(环丁基氨基)-N-(3-(3,4-二氢异喹啉-2(1H)-基)-2-羟丙基)异烟酰胺
(SP-4-1)-二氯双(喹啉)-钯
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(R,S)-可替宁N-氧化物-甲基-d3
(R,S)-六氢-3H-1,2,3-苯并噻唑-2,2-二氧化物-3-羧酸叔丁酯
(R)-(+)-5'-苄氧基卡维地洛
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-卡洛芬
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(R)-4-异丙基-2-恶唑烷硫酮
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(N-{4-[(6-溴-2-氧代-1,3-苯并恶唑-3(2H)-基)磺酰基]苯基}乙酰胺)
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6R,7R)-7-苯基乙酰胺基-3-[(Z)-2-(4-甲基噻唑-5-基)乙烯基]-3-头孢唑啉-4-羧酸二苯甲基酯
(6-羟基嘧啶-4-基)乙酸
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(6,6-二甲基-3-(甲硫基)-1,6-二氢-1,2,4-三嗪-5(2H)-硫酮)
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5R,Z)-3-(羟基((1R,2S,6S,8aS)-1,3,6-三甲基-2-((E)-prop-1-en-1-yl)-1,2,4a,5,6,7,8,8a-八氢萘-1-基)亚甲基)-5-(羟甲基)-1-甲基吡咯烷-2,4-二酮
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-(4-乙氧基-3-甲基苄基)-1,3-苯并二恶茂)
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氯-2,1,3-苯并噻二唑-4-基)-氨基甲氨基硫代甲酸甲酯一氢碘
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(5-氨基-1,3,4-噻二唑-2-基)甲醇
(4aS-反式)-八氢-1H-吡咯并[3,4-b]吡啶
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(4S,4''S)-2,2''-环亚丙基双[4-叔丁基-4,5-二氢恶唑]
(4-(4-氯苯基)硫代)-10-甲基-7H-benzimidazo(2,1-A)奔驰(德)isoquinolin-7一
(4-苄基-2-甲基-4-nitrodecahydropyrido〔1,2-a][1,4]二氮杂)
(4-甲基环戊-1-烯-1-基)(吗啉-4-基)甲酮
(4-己基-2-甲基-4-nitrodecahydropyrido〔1,2-a][1,4]二氮杂)
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)