烯丙基正癸酸酯 | 57856-81-2
中文名称
烯丙基正癸酸酯
中文别名
——
英文名称
allyl decanoate
英文别名
decanoic acid 2-propenyl ester;prop-2-enyl decanoate
CAS
57856-81-2
化学式
C13H24O2
mdl
MFCD00048984
分子量
212.332
InChiKey
DQVOTEHORLHPRW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:272.0±9.0 °C(Predicted)
-
密度:0.878±0.06 g/cm3(Predicted)
-
LogP:5.166 (est)
-
保留指数:1458;1462;1456
-
稳定性/保质期:
在常温常压下保持稳定,应避免接触水分。
计算性质
-
辛醇/水分配系数(LogP):4.8
-
重原子数:15
-
可旋转键数:11
-
环数:0.0
-
sp3杂化的碳原子比例:0.769
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2915900090
-
储存条件:请将产品存放在阴凉、干燥的地方保存。
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:A novel cleavage of allyl protection摘要:A novel, efficient, and mild cleavage of allyl protection is developed employing perfluoroalkylation and subsequent elimination. (C) 1998 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4039(98)00906-x
-
作为产物:描述:1,3-dichloro-2-propyl decanoate 在 sodium iodide 作用下, 以 丁酮 为溶剂, 反应 48.0h, 以91%的产率得到烯丙基正癸酸酯参考文献:名称:串联的Finkelstein重排消除反应:烯丙基酯的直接合成路线摘要:烯丙基酯可以通过NaI诱导的2-氯-1-(氯甲基)乙基酯的Finkelstein重排消除反应获得。碘化钠可以在还原当量以下使用还原剂作为硫代硫酸钠使用。使用大多数已研究的各种酯均可获得高收率。所描述的方法避免了使用烯丙醇作为试剂。2-氯-1-(氯甲基)乙酯是由甘油制成的,甘油是生物柴油工业的主要副产品。还证实了碘作为水解烯丙基酯的试剂的有效性。DOI:10.1016/j.tet.2009.04.042
文献信息
-
[Pd(μ-Br)(P<sup><i>t</i></sup>Bu<sub>3</sub>)]<sub>2</sub> as a Highly Active Isomerization Catalyst: Synthesis of Enol Esters from Allylic Esters作者:Patrizia Mamone、Matthias F. Grünberg、Andreas Fromm、Bilal A. Khan、Lukas J. GooßenDOI:10.1021/ol301563g日期:2012.7.20to be highly active for catalyzing double-bond migration in various substrates such as unsaturated ethers, alcohols, amides, and arenes, under mild conditions. It efficiently mediates the conversion of allylic esters into enol esters, rather than inserting into the allylic C–O bond. The broad applicability of this reaction was demonstrated with the synthesis of 22 functionalized enol esters.发现二聚体Pd(I)络合物[Pd(μ-Br)(P t Bu 3)] 2具有高活性,可催化不饱和醚,醇,酰胺和芳烃等各种底物中的双键迁移,在温和的条件下。它有效地调节了烯丙基酯到烯醇酯的转化,而不是插入烯丙基C–O键。通过合成22种官能化的烯醇酯证明了该反应的广泛适用性。
-
[EN] MANUFACTURE OF AN EPOXYETHYL CARBOXYLATE OR GLYCIDYL CARBOXYLATE<br/>[FR] FABRICATION D'UN CARBOXYLATE D'ÉPOXYÉTHYLE OU D'UN CARBOXYLATE DE GLYCIDYLE申请人:MOMENTIVE SPECIALTY CHEMICALS RES S A公开号:WO2011095294A1公开(公告)日:2011-08-11The invention relates to a process for the manufacture of an epoxyethyl carboxylate or glycidyl carboxylate, including reacting a vinyl carboxylate or an allyl carboxylate using an oxidant and a water-soluble manganese complex in an aqueous reaction medium, and the water-soluble manganese complex comprises an oxidation catalyst, characterized in that the water-soluble manganese complex is a mononuclear species of the general formula (I): [LMnX3]Y, or a binuclear species of the general formula (II): [LMn(μ-X)3MnL]Yn, wherein Mn is a manganese; L is a ligand and each L is independently a polydentate ligand, each X is independently a coordinating species and each μ-X is independently a bridging coordinating species, Y is a non-coordinating counter ion, and wherein the epoxidation is carried out at a pH in the range of from 1.0 to 7.0.
-
Method for the Preparation of Palladium(I) Tri-Tert-Butylphosphine Bromide Dimer and Process for its Use in Isomerization Reactions申请人:Goossen Lukas公开号:US20140187803A1公开(公告)日:2014-07-03The invention provides a new method for the preparation of the dimeric Pd(l) tri-tert.-butylphosphine bromide complex, characterized by the chemical formula [Pd(μ-Br)(P t Bu 3 )] 2 . The method is based on a comproportionation reaction in which a Pd(ll) compound (=PdBr 2 ) is reacted with a Pd(0) compound (=Pd(P t Bu 3 ) 2 ) in organic solvents to yield the [Pd(μ-Br)(P t Bu 3 )] 2 compound having the Pd atoms in the formal oxidation state +1. Unreacted PdBr 2 may be reused in the process. The method is straightforward and applicable for industrial scale production and provides high product yields. Further, a new process for the isomerization of allyl ethers of the general type R 1 —C(O)—O—CH(R 2 )—C(R 3 )═CH 2 employing the compound Pdμ-Br)(P t Bu 3 )] 2 as a catalyst is disclosed.
-
Method for the preparation of palladium(I) tri-tert-butylphosphine bromide dimer and process for its use in isomerization reactions申请人:Goossen Lukas公开号:US09192927B2公开(公告)日:2015-11-24The invention provides a new method for the preparation of the dimeric Pd(l) tri-tert.-butylphosphine bromide complex, characterized by the chemical formula [Pd(μ-Br)(PtBu3)]2. The method is based on a comproportionation reaction in which a Pd(ll) compound (═PdBr2) is reacted with a Pd(0) compound (═Pd(PtBu3)2) in organic solvents to yield the [Pd(μ-Br)(PtBu3)]2 compound having the Pd atoms in the formal oxidation state +1. Unreacted PdBr2 may be reused in the process. The method is straightforward and applicable for industrial scale production and provides high product yields. Further, a new process for the isomerization of allyl ethers of the general type R1—C(O)—O—CH(R2)—C(R3)═CH2 employing the compound Pdμ-Br)(PtBu3)]2 as a catalyst is disclosed.
-
Process for the manufacture of a 1,2-Epoxide申请人:Hexion Specialty Chemicals Research Belgium S.A.公开号:EP2149569A1公开(公告)日:2010-02-03The invention relates to a process for the manufacture of a 1,2-epoxide by catalytic oxidation of a terminal olefin with hydrogen peroxide wherein the catalytic oxidation is performed in a biphasic system comprising an organic phase and an aqueous reaction medium, wherein a water-soluble manganese complex is used as oxidation catalyst, wherein a terminal olefin is used with a solubility at 20°C of at least 0,01 to 100 g in 1 liter water, and wherein the molar ratio of terminal olefin to hydrogen peroxide is in the range of from 1:0.1 to 1:2.
表征谱图
-
氢谱1HNMR
-
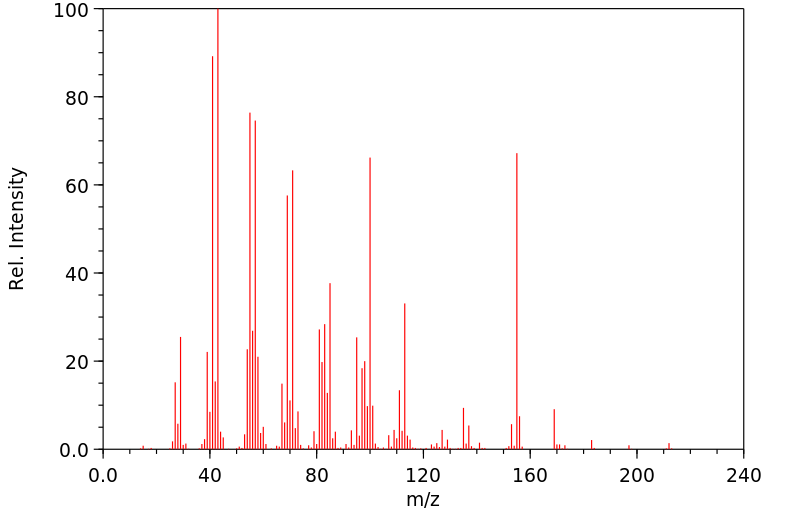
质谱MS
-
碳谱13CNMR
-
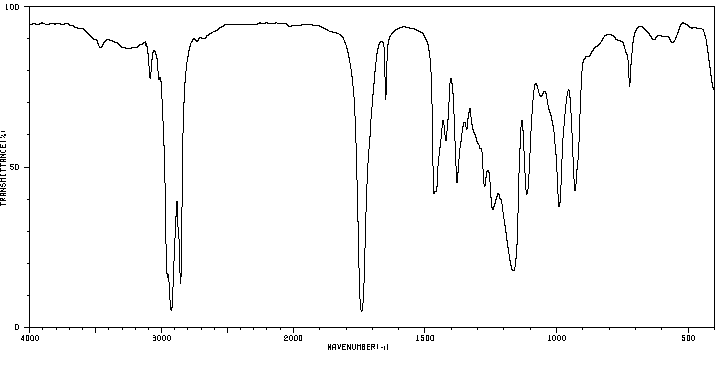
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯