aspartic acid hydrochloride | 40149-75-5
中文名称
——
中文别名
——
英文名称
aspartic acid hydrochloride
英文别名
1,2-Dicarboxyethan-1-aminium chloride;1,2-dicarboxyethylazanium;chloride
CAS
40149-75-5
化学式
C4H7NO4*ClH
mdl
——
分子量
169.565
InChiKey
DWHMPBALQYTJFJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-0.71
-
重原子数:10
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:101
-
氢给体数:4
-
氢受体数:5
反应信息
-
作为反应物:描述:参考文献:名称:Mulengi, J. Kajima; Fatmi, N., Bulletin des Societes Chimiques Belges, 1992, vol. 101, # 4, p. 257 - 260摘要:DOI:
-
作为产物:描述:参考文献:名称:Neopetrosiamides,来自海洋海绵Neopetrosia sp。的肽。抑制了人类肿瘤细胞对类阿米巴的入侵。摘要:[结构:参见正文]从海洋海绵Neopetrosia sp。中分离出了两种新的非对映异构的三环肽,它们能抑制变形虫入侵人类肿瘤细胞,它们分别是Neopetrosiamdes A(1)和B(2)。收集在巴布亚新几内亚。通过分析MS和NMR数据阐明新石油硅酰胺的结构,并通过化学降解证实。DOI:10.1021/ol051524c
文献信息
-
Synthesis of β-cyanoalanine and enantiomerically enriched aspartate derivatives via the Zn- or In-mediated nucleophilic addition to α-imino esters作者:Arya Jayadev Sudha、Nayyar Ahmad Aslam、Akshey Sandhu、Makoto Yasuda、Akio Baba、Srinivasarao Arulananda BabuDOI:10.1016/j.tet.2020.131217日期:2020.64-bromocrotonates to enantiopure N-tert-butanesulfinyl α-imino esters gave the corresponding enantiomerically enriched β-vinyl aspartates 10c-e (aspartic acid derivatives) with high diastereoselectivity (syn isomers). The stereochemistry of the vicinal stereocenters of the compounds 10c-e (major isomers) were assigned based on the X-ray structure of a derivative 11d which was obtained from 10c. To show the我们报道了β-氰基丙氨酸衍生物和对映体富集的天冬氨酸的合成。锌介导的α-溴乙腈加至α-亚氨基酯中,以令人满意的良好产率获得了各种β-氰基丙氨酸衍生物3a-1和5a-d。所述锌或铟-介导的加入α-溴乙腈或2-溴乙酸乙酯于对映体纯ñ -叔-butanesulfinyl α -亚氨基酯,得到相应的产品3M-P在令人满意的产率和非对映选择性。值得注意的是,铟介导的4-溴巴豆酸烷基酯加成对映体ñ -叔-butanesulfinyl α -亚氨基酯,得到相应的对映体富集的β -乙烯基天冬氨酸10C-E (天冬氨酸衍生物)具有高非对映选择性(顺式异构体)。基于衍生自10c的衍生物11d的X射线结构,确定化合物10c-e(主要异构体)的邻近立体中心的立体化学。为了显示β-氰基丙氨酸和β-乙烯基天冬氨酸衍生物的效用,代表性的β-氰基丙氨酸化合物3a / 3b,对5d和天冬氨酸衍生物10c进行选择的合成转化。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
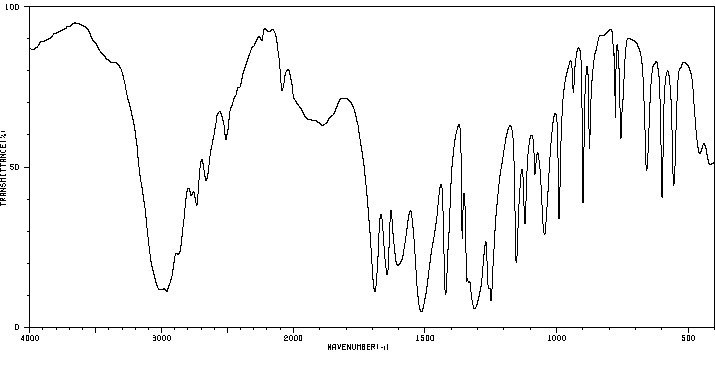
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸