烯丙氧基三甲硅烷 | 18146-00-4
中文名称
烯丙氧基三甲硅烷
中文别名
三甲基烯丙氧硅烷;三甲基(2-丙基氧)硅烷;3-(三甲基硅氧基)丙烯;烯丙氧基三甲基硅烷
英文名称
allyloxytrimethylsilane
英文别名
allyl trimethylsilyl ether;trimethylallyloxysilane;trimethyl(prop-2-enoxy)silane
CAS
18146-00-4
化学式
C6H14OSi
mdl
MFCD00008643
分子量
130.262
InChiKey
MNMVKGDEKPPREK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:<0°C
-
沸点:100-102 °C(lit.)
-
密度:0.773 g/mL at 25 °C(lit.)
-
闪点:31 °F
-
保留指数:708.5
-
稳定性/保质期:
- 高度易燃,请勿在使用现场吸烟。
计算性质
-
辛醇/水分配系数(LogP):2.31
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险等级:3.1
-
危险品标志:F,Xi
-
安全说明:S16,S26,S36
-
危险类别码:R11
-
WGK Germany:3
-
海关编码:2931900090
-
危险品运输编号:UN 1993 3/PG 2
-
危险类别:3.1
-
包装等级:II
-
危险性防范说明:P210,P235,P240,P241,P242,P243,P260,P264,P280,P301+P330+P331,P303+P361+P353,P304+P340,P305+P351+P338,P310,P321,P363,P370+P378,P403+P235,P405,P501
-
危险性描述:H225,H314
-
储存条件:应将氩气密封储存于0至4℃的通风良好之处。
SDS
模块 1. 化学品
1.1 产品标识符
: 烯丙氧基三甲基硅烷
产品名称
1.2 鉴别的其他方法
3-(Trimethylsiloxy)propene
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
易燃液体 (类别 2)
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
特异性靶器官系统毒性(一次接触) (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H225 高度易燃液体和蒸气
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P210 远离热源、火花、明火和热表面。- 禁止吸烟。
P233 保持容器密闭。
P240 容器和接收设备接地/等势连接。
P241 使用防爆的电气/ 通风/ 照明 设备。
P242 只能使用不产生火花的工具。
P243 采取防止静电放电的措施。
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 戴防护手套/穿防护服/戴护目镜/戴面罩.
响应
P303 + P361 + P353 如皮肤(或头发)沾染:立即去除/ 脱掉所有沾染的衣服。用水清洗皮肤/
淋浴。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾染的衣服,清洗后方可重新使用。
P370 + P378 火灾时: 用干的砂子,干的化学品或耐醇性的泡沫来灭火。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P403 + P235 存放在通风良好的地方。保持低温。
P405 存放处须加锁。
处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 3-(Trimethylsiloxy)propene
别名
: C6H14OSi
分子式
: 130.26 g/mol
分子量
组分 浓度或浓度范围
AllyloxytrimethylsiLAne
-
化学文摘登记号(CAS 18146-00-4
No.) 242-031-4
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
禁止催吐。 切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
小(起始)火时,使用媒介物如“乙醇”泡沫、干化学品或二氧化碳。大火时,尽可能使用水灭火。使用大量(
洪水般的)水以喷雾状应用;水柱可能是无效的。用大量水降温所有受影响的容器。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 二氧化硅
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
用水喷雾冷却未打开的容器。
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 移去所有火源。
人员疏散到安全区域。 谨防蒸气积累达到可爆炸的浓度。蒸气能在低洼处积聚。
6.2 环境保护措施
如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
围堵溢出,用防电真空清洁器或湿刷子将溢出物收集起来,并放置到容器中去,根据当地规定处理(见第13部
分)。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免吸入蒸气和烟雾。
切勿靠近火源。-严禁烟火。采取措施防止静电积聚。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
对湿度敏感
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 阻燃防静电防护服,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 透明, 液体
颜色: 无色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 沸点、初沸点和沸程
100 - 102 °C - lit.
g) 闪点
1 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
0.773 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
热,火焰和火花。 极端温度和直接日晒。
10.5 不相容的物质
强酸, 强碱, 强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
在装备有加力燃烧室和洗刷设备的化学焚烧炉内燃烧处理,特别在点燃的时候要注意,因为此物质是高度易燃
性物质 将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 1993 国际海运危规: 1993 国际空运危规: 1993
14.2 联合国运输名称
欧洲陆运危规: FLAMMABLE LIQUID, N.O.S. (AllyloxytrimethylsiLAne)
国际海运危规: FLAMMABLE LIQUID, N.O.S. (AllyloxytrimethylsiLAne)
国际空运危规: FLAMMAble liquid, n.o.s. (AllyloxytrimethylsiLAne)
14.3 运输危险类别
欧洲陆运危规: 3 国际海运危规: 3 国际空运危规: 3
14.4 包裹组
欧洲陆运危规: II 国际海运危规: II 国际空运危规: II
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Ein neues Verfahren zur Überführung von Alkyl-silylethern in Alkylbromide unter schonenden Bedingungen摘要:DOI:10.1055/s-1982-29802
-
作为产物:参考文献:名称:Synthesis of diosphenol ethers by means of alkoxytrimethylsilanes摘要:DOI:10.1016/s0040-4039(00)85105-9
-
作为试剂:描述:2-碘噻吩 在 烯丙氧基三甲硅烷 、 palladium diacetate 、 lithium chloride 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 5.0h, 以30%的产率得到(2E)-3-(2-噻吩基)丙烯醛参考文献:名称:钯促进的烯丙基三甲基甲硅烷基醚与芳基碘的反应摘要:烯丙基三甲基甲硅烷基醚在乙酸钯和氯化锂存在下与芳基碘反应,选择性地得到 β-芳基 (E)-α,β-不饱和羰基化合物。DOI:10.1246/cl.1981.403
文献信息
-
Preparation of Nano Silica Supported Sodium Hydrogen Sulfate: As an Efficient Catalyst for the Trimethyl, Triethyl and<i>t</i>-Butyldimethyl Silylations of Aliphatic and Aromatic Alcohols in Solution and under Solvent-free Conditions作者:Abdolreza Abri、Somayeh RanjdarDOI:10.1002/jccs.201300586日期:2014.8Nano silica supported sodium hydrogen sulfate has been prepared by mixing NaHSO4 with activated Nano silicagel. We wish to report a new method for the synthesis of trimethyl (TMS), triethyl (TES) and t‐butyldimethyl silyl (TBS) ethers from benzylic, allylic, propargylic alcohols, phenols, naphtholes and some of phenolic drugs in the solution and under solvent‐free conditions.
-
Novel Nickel-Catalyzed Coupling Reaction of Allyl Ethers with Chlorosilanes, Alkyl Tosylates, or Alkyl Halides Promoted by Vinyl-Grignard Reagent Leading to Allylsilanes or Alkenes作者:Jun Terao、Hiroyasu Watabe、Hiroyuki Watanabe、Nobuaki KambeDOI:10.1002/adsc.200404192日期:2004.12method for a carbon-silicon or carbon-carbon bond forming reaction between allyl ethers and chlorosilanes, alkyl tosylates, or alkyl halides giving rise to allylsilanes or alkenes has been developed. This reaction proceeds efficiently at ambient temperature by the combined use of nickel catalysts and a vinyl-Grignard reagent. A possible reaction pathway involving the formation of allyl-Grignard reagents
-
Cobalt and Manganese Salts of p -Aminobenzoic Acid Supported on Silica Gel: A Versatile Catalyst for Oxidation by Molecular Oxygen作者:Mohammed M. Hashemi、Yusef AhmadibeniDOI:10.1007/s00706-002-0534-3日期:2003.2.1A 1:1 molar ratio of the cobalt and manganese salts of p -amino benzoic acid supported on silica gel is an effective catalyst for the oxidation of various organic compounds in reasonable yields using molecular oxygen. The catalyst can be reused several times.
-
Efficient and practical protocol for silylation of hydroxyl groups using reusable lithium perchlorate dispread in silica gel under neutral condition作者:Najmedin Azizi、Rozbeh Yousefi、Mohammad R. SaidiDOI:10.1016/j.jorganchem.2005.11.005日期:2006.2for the trimethylsilylation of a wide variety of alcohols, including primary, allylic, benzylic, secondary, hindered secondary, tertiary, and phenols with hexamethyldisilazane on the surface of silica gel dispersed with LiClO4 in room temperature at few minutes in excellent yields under neutral conditions is reported. This procedure also allows the excellent selectivity under LP-SiO2 system for silylation
-
Aluminium Triflate [Al(OTf)<sub>3</sub>] as a Recyclable Catalyst for the Conversion of α-Hydroxyphosphonates, Alcohols and Phenols to Their Corresponding O-Silylated Products with Hexamethyldisilazane (HMDS)作者:Habib Firouzabadi、Nasser Iranpoor、Sara Sobhani、Soheila GhassamipourDOI:10.1055/s-2005-861789日期:——Al(OTf)3 as a recyclable catalyst conducts the efficient conversion of various types of α-hydroxyphosphonates to their corresponding α-trimethysilyloxyphosphonates with HMDS in the absence of solvent at room temperature. The general applicability of the catalyst under solvent-free conditions is demonstrated by applying it for the successful silylation of alcohols and phenols with HMDS in high yields.
表征谱图
-
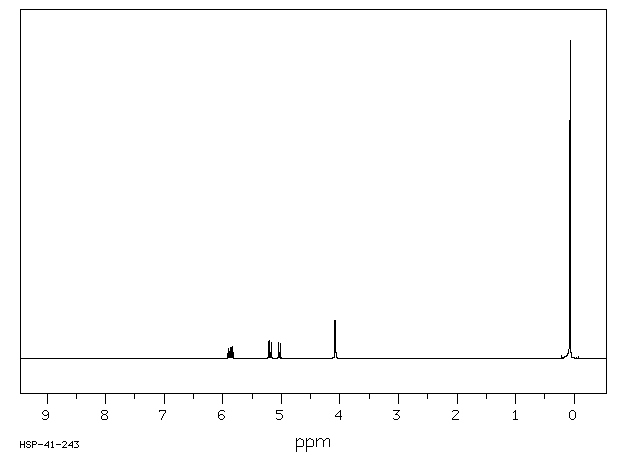
氢谱1HNMR
-
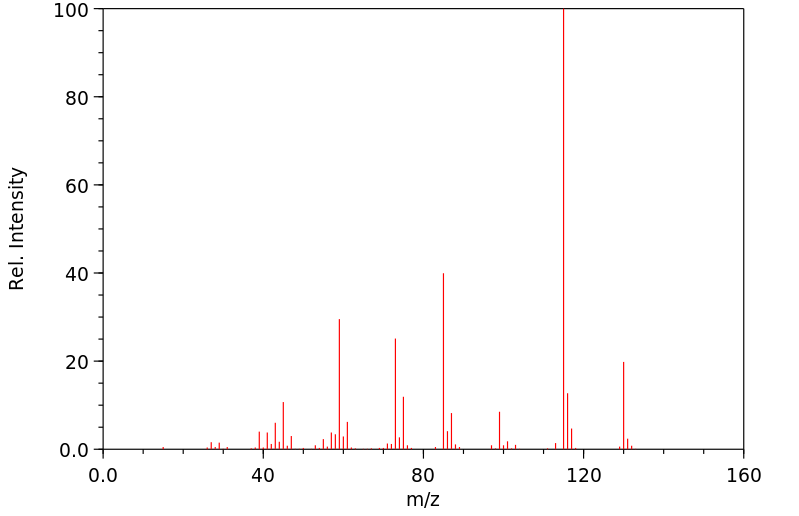
质谱MS
-
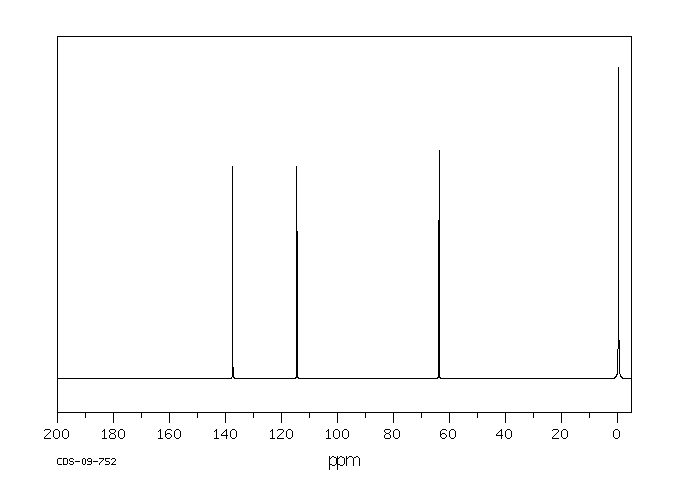
碳谱13CNMR
-
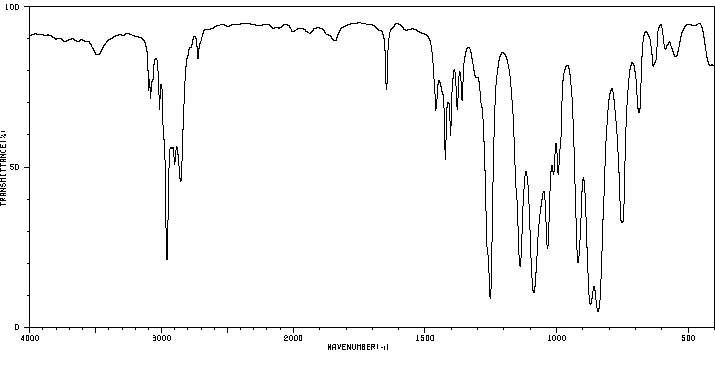
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-溴乙氧基)-特丁基二甲基硅烷
鲸蜡基聚二甲基硅氧烷
骨化醇杂质DCP
马沙骨化醇中间体
马来酸双(三甲硅烷)酯
顺式-二氯二(二甲基硒醚)铂(II)
顺-N-(1-(2-乙氧基乙基)-3-甲基-4-哌啶基)-N-苯基苯酰胺
降钙素杂质13
降冰片烯基乙基三甲氧基硅烷
降冰片烯基乙基-POSS
间-氨基苯基三甲氧基硅烷
镓,二(1,1-二甲基乙基)甲基-
镁,氯[[二甲基(1-甲基乙氧基)甲硅烷基]甲基]-
锑,二溴三丁基-
铷,[三(三甲基甲硅烷基)甲基]-
铂(0)-1,3-二乙烯-1,1,3,3-四甲基二硅氧烷
钾(4-{[二甲基(2-甲基-2-丙基)硅烷基]氧基}-1-丁炔-1-基)(三氟)硼酸酯(1-)
金刚烷基乙基三氯硅烷
酰氧基丙基双封头
达格列净杂质
辛醛,8-[[(1,1-二甲基乙基)二甲基甲硅烷基]氧代]-
辛甲基-1,4-二氧杂-2,3,5,6-四硅杂环己烷
辛基铵甲烷砷酸盐
辛基衍生化硅胶(C8)ZORBAX?LP100/40C8
辛基硅三醇
辛基甲基二乙氧基硅烷
辛基三甲氧基硅烷
辛基三氯硅烷
辛基(三苯基)硅烷
辛乙基三硅氧烷
路易氏剂-3
路易氏剂-2
路易士剂
试剂Cyanomethyl[3-(trimethoxysilyl)propyl]trithiocarbonate
试剂3-[Tris(trimethylsiloxy)silyl]propylvinylcarbamate
试剂3-(Trimethoxysilyl)propylvinylcarbamate
试剂2-(Trimethylsilyl)cyclopent-2-en-1-one
试剂11-Azidoundecyltriethoxysilane
西甲硅油杂质14
衣康酸二(三甲基硅基)酯
苯胺,4-[2-(三乙氧基甲硅烷基)乙基]-
苯磺酸,羟基-,盐,单钠聚合甲醛,1,3,5-三嗪-2,4,6-三胺和脲
苯甲醇,a-[(三苯代甲硅烷基)甲基]-
苯并磷杂硅杂英,5,10-二氢-10,10-二甲基-5-苯基-
苯基二甲基氯硅烷
苯基二甲基乙氧基硅
苯基二甲基(2'-甲氧基乙氧基)硅烷
苯基乙酰氧基三甲基硅烷
苯基三辛基硅烷
苯基三甲氧基硅烷