N-phenylacetyl-L-glutamic acid | 2752-33-2
中文名称
——
中文别名
——
英文名称
N-phenylacetyl-L-glutamic acid
英文别名
N-Phenylacetyl-L-glutaminsaeure;N-Phenylacetyl-L-glutaminsaeure;N-Phenylacetylglutamic acid;(2S)-2-[(2-phenylacetyl)amino]pentanedioic acid
CAS
2752-33-2
化学式
C13H15NO5
mdl
MFCD16378666
分子量
265.266
InChiKey
PTSRBZOZSRJCKX-JTQLQIEISA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:19
-
可旋转键数:7
-
环数:1.0
-
sp3杂化的碳原子比例:0.307
-
拓扑面积:104
-
氢给体数:3
-
氢受体数:5
SDS
反应信息
-
作为反应物:参考文献:名称:255. N-(N-phenylacetyl-α-
DL -glutamyl)-D -penicillamine摘要:DOI:10.1039/jr9510001143 -
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 barium dihydroxide 作用下, 生成 N-phenylacetyl-L-glutamic acid参考文献:名称:Thierfelder; Sherwin, Chemische Berichte, 1914, vol. 47, p. 2632摘要:DOI:
文献信息
-
Compositions for and uses thereof for treating viral disease with phenylacetate and derivatives thereof申请人:THE GOVERNMENT OF THE UNITED STATES OF AMERICA, as represented by THE SECRETARY OF THE DEPARTMENT OF HEALTH AND HUMAN SERVICES公开号:EP1108428A2公开(公告)日:2001-06-20A pharmaceutical composition for inhibiting viral replication and spread comprises a pharmaceutically effective amount of a compound of formula I: R1R2CH-COOH wherein R1 is selected from H, CH3, CH3-O-, C2H5 or C3H7; R2 is selected from aryl X is wherein X is independently selected from halogen or hydroxy, and n is 0 to 4; a stereoisomer thereof, or a pharmaceutically acceptable salt thereof, and mixtures thereof together with a pharmaceutically acceptable carrier.
-
Compositions for and uses thereof for altering gene expression with phenylacetate and derivatives thereof申请人:THE GOVERNMENT OF THE UNITED STATES OF AMERICA, as represented by THE SECRETARY OF THE DEPARTMENT OF HEALTH AND HUMAN SERVICES公开号:EP1108427A2公开(公告)日:2001-06-20A pharmaceutical composition for inhibiting altering gene expression, inducing differentiation in nonmalignant mammalian cells, and for the treatment of β-chain hemoglobinopathies, comprises a pharmaceutically effective amount of a compound of formula I: R1R2CH-COOH wherein R1 is selected from H, CH3, CH3-O-, C2H5 or C3H7; R2 is selected from X is wherein X is independently selected from halogen or hydroxy, and n is 0 to 4; a stereoisomer thereof, or a pharmaceutically acceptable salt thereof, and mixtures thereof together with a pharmaceutically acceptable carrier.
-
Antiviral composition comprising phenylacetic acid derivatives申请人:THE GOVERNMENT OF THE UNITED STATES OF AMERICA, as represented by THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES公开号:EP1484059A2公开(公告)日:2004-12-08A pharmaceutical composition for inhibiting viral replication and spread comprises a pharmaceutically effective amount of a compound of formula I: R1R2CH-COOH wherein R1 is selected from H, CH3, CH3-O-, C2H5 or C3H7; R2 is selected from aryl (formulae of original claim 6) wherein X is independently selected from halogen or hydroxy, and n is 0 to 4; a stereoisomer thereof, or a pharmaceutically acceptable salt thereof, and mixtures thereof together with a pharmaceutically acceptable carrier.
-
Pharmaceutical composition for inhibiting altering gene expression and for inducing differentiation in non-malignant cells申请人:THE GOVERNMENT OF THE UNITED STATES OF AMERICA, as represented by THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES公开号:EP1484058A2公开(公告)日:2004-12-08A pharmaceutical composition for inhibiting altering gene expression, inducing differentiation in nonmalignant mammalian cells, and for the treatment of β-chain hemoglobinopathies, comprises a pharmaceutically effective amount of a compound of formula I: R1R2CH-COOH wherein R1 is selected from H, CH3, CH3-O-, C2H5 or C3H7; R2 is selected from wherein X is independently selected from halogen or hydroxy, and n is 0 to 4; a stereoisomer thereof, or a pharmaceutically acceptable salt thereof, and mixtures thereof together with a pharmaceutically acceptable carrier.
-
Preparation of d-amino acids by enzymatic kinetic resolution using a mutant of penicillin-G acylase from E. coli作者:Chiara Carboni、Hans G.T. Kierkels、Lucia Gardossi、Kamil Tamiola、Dick B. Janssen、Peter J.L.M. QuaedfliegDOI:10.1016/j.tetasy.2005.12.023日期:2006.1We have demonstrated for the first time that D-glutamine (D-Gln) and D-glutamic acid (D-Glu) can be efficiently obtained in high ee (97% and 90%, respectively) by enzymatic kinetic resolution of D,L-Gln and D,L-Glu. This was achieved by enantioselective conversion of the L-enantiomers to their N-phenylacetyl derivatives in aqueous solution, using a mutant of penicillin-G acylase (PGA) from E coli and phenylacetic acid methylester as the acyl donor. Kinetic modeling studies suggest that the high ee values obtained are both due to a strong enantiopreference for the L-amino acid in the deacylation step of the covalent enzyme intermediate, as well as to completeness of conversion that is transiently obtained as a result of the distinct preference of the mutant PGA for phenylacetic acid methylester over the N-phenylacetyl-L-amino acid product. For the other amino acids tested (Asn, Asp, and Ser), the highest ee values that were obtained for the remaining D-enantiomer are moderate (50-80%)because of lower enantioselectivity in the enzyme deacylation step and due to less complete conversion of the L-amino acid caused by competition for the active site between the acyl donor and the N-phenylacetyl-L-amino acid that is produced. The results demonstrate that the mutated PGA has great potential for the production of optically active D-amino acids by kinetic resolution. (c) 2006 Published by Elsevier Ltd.
表征谱图
-
氢谱1HNMR
-
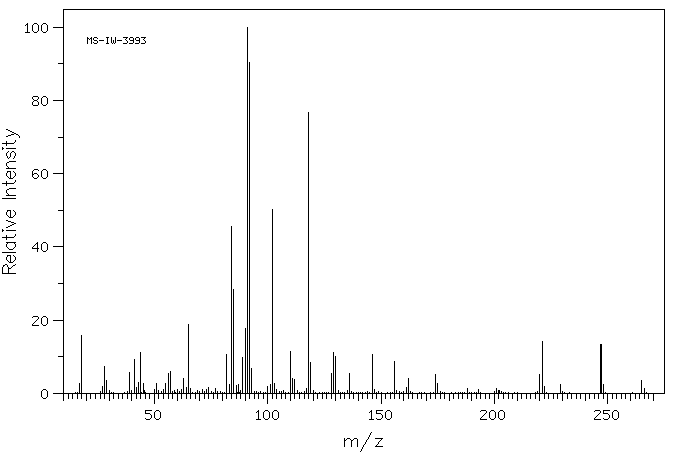
质谱MS
-
碳谱13CNMR
-
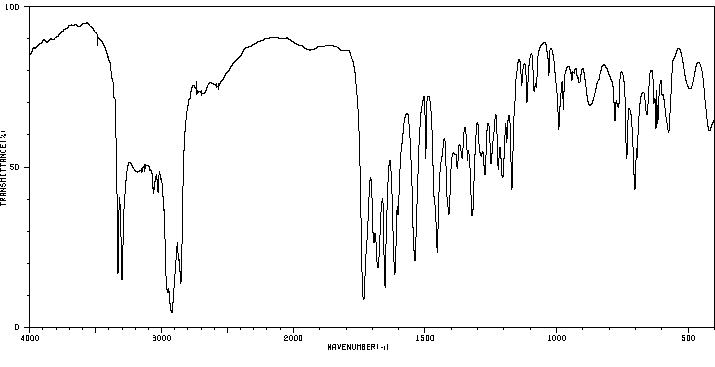
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸