6-(2-phenylethenyl)fulvene | 32174-25-7
中文名称
——
中文别名
——
英文名称
6-(2-phenylethenyl)fulvene
英文别名
[(1E)-3-cyclopenta-2,4-dien-1-ylideneprop-1-en-1-yl]benzene;[(E)-3-cyclopenta-2,4-dien-1-ylideneprop-1-enyl]benzene
CAS
32174-25-7
化学式
C14H12
mdl
——
分子量
180.249
InChiKey
XYJUKARVJMWHBT-IZZDOVSWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:329.4±9.0 °C(Predicted)
-
密度:1.116±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:14
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
反应信息
-
作为反应物:描述:6-(2-phenylethenyl)fulvene 以 环己烷 、 甲苯 为溶剂, 反应 2.0h, 生成 6-tert-butyl-1-(3-phenyl-allylidene)-1H-indene参考文献:名称:6芳基烯基富烯与二烯和亲二烯体的Diels-Alder反应及相关化学摘要:已经表明6-(2-苯基乙烯基)富烯烯以二烯和亲二烯体的形式参与Diels-Alder反应,并且已经完成了导致广泛共轭的苯并富烯酮的双环[2.2.2]-辛二酮的光化学转化。DOI:10.1016/s0040-4020(97)10160-0
-
作为产物:参考文献:名称:Scope and Limitations of Fulvene Syntheses. Preparation of 6-Vinyl-Substituted and -Functionalized Fulvenes. First Examples of Nucleophilic Substitution on a 6-(Chloromethyl)fulvene摘要:Very few 6-vinylfulvenes have previously been reported in the Literature. In a few cases where Little's procedure (using pyrrolidine as base) has been employed, most enones undergo conjugate attack by the cyclopentadienyl anion followed by either a retroaldol reaction or dihydropentalene formation. In several cases, Diels-Alder reaction of the enone with cyclopentadiene occurs rather than condensation. We have found that in cases where the Little procedure fails to give the desired 6-vinylfulvenes, the Thiele method using NaOH (or NaOMe in some cases) as base gives satisfactory results. In the latter instances, Michael attack is completely suppressed in all but one example. By appropriate choice of base, a variety of fulvenes carrying functional groups on the 6-alkyl position can be prepared. Some of these fulvenes have been shown to undergo further functional group transformations (e.g., nucleophilic substitutions); giving rise to derivatives bearing SR, S(O)R, N-3 or SCN groups.DOI:10.1021/jo00109a010
文献信息
-
Catalytic asymmetric [2+2] cycloaddition between quinones and fulvenes and a subsequent stereoselective isomerization to 2,3-dihydrobenzofurans作者:Haifeng Zheng、Chaoran Xu、Yan Wang、Tengfei Kang、Xiaohua Liu、Lili Lin、Xiaoming FengDOI:10.1039/c7cc03211k日期:——The catalytic enantioselective [2+2] cycloaddition between quinones and fulvenes was achieved, for the first time, by the use of a chiral copper(II) complex catalyst. The transformation afforded a series of enantiomerically enriched [6,4,5]-tricyclic cyclobutane derivatives in good yields with excellent regio- and stereoselectivities. Furthermore, the [2+2] adducts could be easily converted into formal
-
1,3-Dipolar Cycloaddition Reaction of Nitrile N-Oxides to 6-(2-Phenylethenyl)fulvene
-
Hetero-Diels-Alder Reaction of 3-Bromo-7-(bromomethyl)tetracyclo[5.3.1.0<sup>2,6</sup>.0<sup>4,8</sup>]undec-10(12)-ene-9,11-dione with Pentafulvenes: Facile Synthesis of Novel Polycyclic Cage Compounds Having a Pyran Ring作者:Mangalam Nair、Beena James、E. SureshDOI:10.1055/s-2006-956481日期:2006.12The enone moiety of 3-bromo-7-(bromomethyl)tetracyclo[5.3.1.02,6.04,8]undec-10(12)-ene-9,11-dione undergoes facile hetero-Diels-Alder reaction with pentafulvenes leading to novel cage systems with pyran moiety
-
Diels-Alder Reactions of a 6-Arenyl Fulvene Participating both as Diene and Dienophile作者:Vijay Nair、Anilkumar Nair、K. Radhakrishnan、M. Nandakumar、Nigam RathDOI:10.1055/s-1997-5763日期:1997.7Diels-Alder reactions of 6-(2-phenylethenyl)fulvene with electron deficient dienophiles and dienes are described. The participation of the fulvene both as 4π and 2π addend is rationalised using MNDO and AM1 calculations.
-
[4+2] Cycloaddition reactions of o-thioquinones with pentafulvenes: efficient synthesis of benzoxathiins作者:Vijay Nair、Bini Mathew、Rajeev S Menon、Saumini Mathew、M Vairamani、S PrabhakarDOI:10.1016/s0040-4020(02)00263-6日期:2002.4o-Thioquinones undergo [4+2] cycloaddition reactions with pentafulvenes leading to 1,4-benzoxathiins. Reactions of 6-styrenylfulvene with o-thioquinones also afforded similar products. (C) 2002 Published by Elsevier Science Ltd.
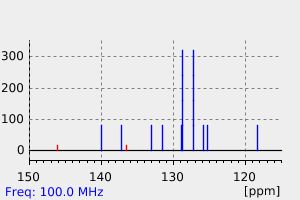
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫