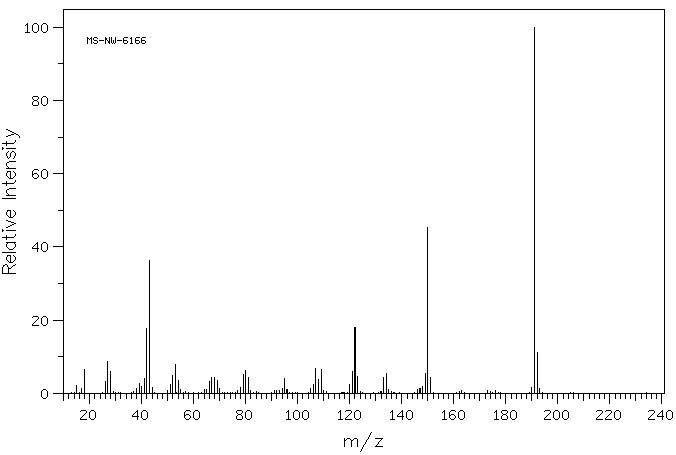
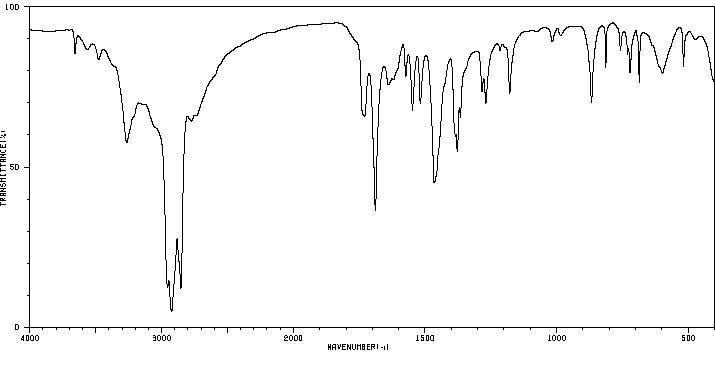
2-氨基-6,7-二甲基-4-羟基他啶水合物 | 611-55-2
中文名称
2-氨基-6,7-二甲基-4-羟基他啶水合物
中文别名
2-氨基-6,7-二甲基-4-羟基蝶啶
英文名称
6,7-Dimethylpterin
英文别名
2-amino-6,7-dimethyl-4(3H)-pteridinone;2-amino-6,7-dimethyl-3H-pteridin-4-one;2-Amino-6,7-dimethyl-3H-pteridin-4-on;2-amino-6,7-dimethyl-4-hydroxypteridine;2-amino-6,7-dimethylpteridin-4-ol;2-amino-6,7-dimethyl-3H-pteridin-4-one
CAS
611-55-2
化学式
C8H9N5O
mdl
MFCD02730134
分子量
191.192
InChiKey
ZKWZUPPXTCQQJL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>300 °C(lit.)
-
沸点:339.1±25.0 °C(Predicted)
-
密度:1.65±0.1 g/cm3(Predicted)
-
溶解度:可溶于酸性水溶液(轻微),碱水溶液(轻微,加热)
-
稳定性/保质期:
性质与稳定性:
常温常压下,不会分解。
计算性质
-
辛醇/水分配系数(LogP):-0.6
-
重原子数:14
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:93.3
-
氢给体数:2
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2933990090
SDS
| Name: | 2-Amino-6 7-dimethyl-4-pteridinol 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 611-55-2 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 611-55-2 | 2-Amino-6,7-dimethyl-4-pteridinol | 97% | 210-270-3 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 611-55-2: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: > 300 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H9N5O
Molecular Weight: 191.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 611-55-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Amino-6,7-dimethyl-4-pteridinol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 611-55-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 611-55-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 611-55-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-azido-6,7-dimethyl-4(3H)-pteridinone 604003-15-8 C8H7N7O 217.19 —— 2-(benzylsulfanyl)-6,7-dimethyl-4(3H)-pteridinone 606964-89-0 C15H14N4OS 298.368 双肼酞嗪 6,7-dimethyl-2,4-pteridine-2,4-diamine 1425-63-4 C8H10N6 190.208 —— 4-(benzylsulfanyl)-6,7-dimethyl-2-pteridinamine 606964-90-3 C15H15N5S 297.384 —— 6,7-dimethyl-4-[(4-methylphenyl)sulfanyl]-2-pteridinamine 606964-91-4 C15H15N5S 297.384 —— 6,7-dimethyl-4-trimethylsiloxy-2-trimethylsilylaminopteridine 36972-94-8 C14H25N5OSi2 335.556 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 6-Methylpterin-7-carbonsaeure 59776-35-1 C8H7N5O3 221.175 —— Pterin-6,7-dicarbonsaeure 59743-03-2 C8H5N5O5 251.158 —— N-(3,4-dihydro-6,7-dimetyl-4-oxopteridin-2-yl)acetamide 19962-31-3 C10H11N5O2 233.23 —— 6,7-dimethyl-4-trimethylsiloxy-2-trimethylsilylaminopteridine 36972-94-8 C14H25N5OSi2 335.556
反应信息
-
作为反应物:描述:参考文献:名称:A Proposed Structure for Folinic Acid-SF, a Growth Factor Derived from Pteroylglutamic Acid摘要:DOI:10.1021/ja01151a074
-
作为产物:描述:4-(benzylsulfanyl)-6,7-dimethyl-2-pteridinamine 在 二甲基二环氧乙烷 、 水 作用下, 以 丙酮 为溶剂, 反应 0.92h, 以90%的产率得到2-氨基-6,7-二甲基-4-羟基他啶水合物参考文献:名称:蝶啶和相关杂环化合物的原型固相合成。摘要:描述了包括蝶啶,嘌呤和脱氮嘌呤的双环多氮杂杂环的通用固相合成的开发。该策略包括将预先形成的嘧啶通过2或4位的硫醚连接至聚苯乙烯树脂,第二环的环化以及通过亲核取代将产物从树脂中直接或氧化裂解。这不仅提供了第二个环中取代基的变化,还提供了切割位点的变化。该方法范围的局限性是由嘧啶基2-或4-硫醚的固有反应性决定的,尽管它们在C5处容易硝化,却难以烷基化和酰化。DOI:10.1039/b300798g
文献信息
-
Dealkylation of quaternary ammonium salts by thiolate anions: A model of the cobalamin-independent methionine synthase reaction.作者:Ellen Hilhorst、Tjoe B.R.A. Chen、Atef S. Iskander、Upendra K. PanditDOI:10.1016/s0040-4020(01)85267-4日期:1994.1The reactions of thiolate ions derived from thiophenol and homocysteine with substituted quaternary ammonium salts result in alkyl transfer from nitrogen to sulfur. A radical mechanism for this transalkylation, accounts for the reactivity pattern of the substrate salts. In a model study of the cobalamin-independent methionine synthase reaction, 5,5,6,7-tetramethyl-5,6,7,8-tetrahydropteridinium salt
-
Stability of 7,8-Dihydropterins in Air-Equilibrated Aqueous Solutions作者:M. Laura Dántola、Mariana Vignoni、Alberto L. Capparelli、Carolina Lorente、Andrés H. ThomasDOI:10.1002/hlca.200890046日期:2008.36-Substituted 7,8-dihydropterins (=2-amino-7,8-dihydropteridin-4(1H)-ones) are heterocyclic compounds that occur in a wide range of living systems and participate in relevant biological functions. In air-equilibrated aqueous solutions, these compounds react with dissolved O2 (autooxidation). The rates of these reactions as well as the products formed strongly depend on the chemical structure of the6-取代的7,8-二氢蝶呤(= 2-氨基-7,8-二氢蝶呤-4(1 H)-ones)是杂环化合物,存在于多种生物系统中,并参与相关的生物学功能。在空气平衡的水溶液中,这些化合物与溶解的O 2反应(自氧化)。这些反应的速率以及形成的产物强烈取决于取代基的化学结构。带有电子供体基团作为取代基的7,8-二氢-6-甲基蝶呤和7,8-二氢-6,7-二甲基蝶呤是最活泼的衍生物,并经过蝶呤部分的氧化反应生成相应的氧化衍生物(6-甲基蝶呤和6,7-二甲基蝶呤)。7,8-二氢生物蝶呤,7,8-二氢蝶呤和7,8-二氢叶酸的氧化较慢,它们产生的主要产物为7,8-二氢黄蝶呤。7,8-二氢黄an呤,6-甲酰基-7,8-二氢蝶呤和Sepaapterin相当稳定,并且它们在空气平衡溶液中的消耗在几天之内可以忽略不计。这些化合物与O之间的反应的拟一级反应速率常数2在25℃和40被报道°。还讨论了所得结果的生物学含义。
-
Identification of a Cyclic Nucleotide as a Cryptic Intermediate in Molybdenum Cofactor Biosynthesis作者:Bradley M. Hover、Anna Loksztejn、Anthony A. Ribeiro、Kenichi YokoyamaDOI:10.1021/ja401781t日期:2013.5.8(cPMP). In bacteria, MoaA and MoaC catalyze this transformation, although the specific functions of these enzymes were not fully understood. Here, we report the first isolation and structural characterization of a product of MoaA. This molecule was isolated under anaerobic conditions from a solution of MoaA incubated with GTP, S-adenosyl-L-methionine, and sodium dithionite in the absence of MoaC. Structural钼辅因子 (MOco) 是一种存在于所有生命王国中的氧化还原辅因子,其生物合成对于包括人类在内的许多生物的生存至关重要。MOco 生物合成的第一步是将鸟苷 5'-三磷酸 (GTP) 独特地转化为环状吡喃蝶呤单磷酸 (cPMP)。在细菌中,MOaA 和 MOaC 催化这种转化,尽管这些酶的具体功能尚不完全清楚。在这里,我们报告了 MOaA 产品的首次分离和结构表征。该分子是在厌氧条件下从 MOaA 溶液中分离出来的,该溶液与 GTP、S-腺苷-L-甲硫氨酸和连二亚硫酸钠一起在没有 MOaC 的情况下孵育。通过化学衍生化、MS 和 NMR 光谱的结构表征表明该分子的结构为 (8S)-3',8-cyclo-7,8-二氢鸟苷 5'-三磷酸 (3',8-cH2GTP)。分离的 3',8-cH2GTP 被 MOaC 或其人类同源物 MOCS1B 转化为 cPMP,具有高特异性(Km < 0.060 μM
-
Pteridines, Part CXIII作者:Qizheng Yao、Wolfgang PfleidererDOI:10.1002/hlca.200390025日期:2003.1compounds can be alkylated under Mitsunobu conditions to form from N2-acylpterins (see 2 and 3) and their derivatives (see 5, 6, 8, 9, 11, 13, 15, and 17) selectively the O4-alkyl derivatives 22–31, whereas the electron-donating [(dimethylamino)methyleneamino function in 46–51 gives, in a selective reaction, the N(3)-substitution (52–61). N2,N2-Dimethylpterins and 18 and 19 and N2-methylpterins 20 and 21蝶呤的低溶解度可通过N 2-酰化或形成N 2 -[((二甲基氨基)亚甲基]衍生物而大大改善。这两种类型的化合物可以下被烷基化的Mitsunobu条件从到形式Ñ 2 -acylpterins(参照2和3)和它们的衍生物(参见图5,6,8,9,11,13,15,和17)选择性地将Ò 4 -烷基衍生物22 – 31,而给电子体[[(二甲基氨基)亚甲基氨基]在46 – 51在选择性反应中给出N(3)取代(52 – 61)。Ñ 2,Ñ 2个-Dimethylpterins和18个19和Ñ 2 -methylpterins 20和21直接烷基化还向Ò 4位上(32 - 35,38和39)。脱酰可以在非常温和的条件下通过溶剂解用MeOH(实现22 40,26 41),和所述的位移Ò 4- [2-(4-硝基苯基)乙基]基团在室温下,以相应的蝶啶-2,4-二胺氨前进42 - 45。N 2 -[((二甲基氨基)亚甲基]基团的裂解可很好地与氨(62
-
A traceless solid-phase synthesis of pteridines作者:Colin L. Gibson、Salvatore La Rosa、Colin J. SucklingDOI:10.1016/s0040-4039(02)02782-x日期:2003.2The linking of pyrimidines to polystyrene supports via either a 2- or 4-thioether provides access to pteridines through solid-phase synthesis. Oxidative cleavage (dimethyldioxirane) followed by nucleophilic substitution by amines, azide, or water completes a traceless synthesis of pteridines.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄素酰色氨酸
高蝶酸
骏河毒素
酵母粉
诺米林酸17-β-D-吡喃葡萄糖苷
蝶酸
蝶啶3-氧化物
蝶啶-6-基-甲醇
蝶啶-4,6-二胺
蝶啶-2,4-二胺
蝶呤-6-羧酸
苯癸酸,2-羟基-3,4-二甲氧基-6-甲基
苯并[g]蝶啶-4a(2H)-基,5-乙基-3,4,5,10-四氢-3,7,8,10-四甲基-2,4-二羰基-
苯并[g]蝶啶-2,4(1H,3H)-二酮,5-乙酰基-5,10-二氢-1,3-二甲基-
苯并[g]蝶啶-2,4(1H,3H)-二酮,5,10-二氢-7,8-二甲基-
苯并[g]蝶啶-2,4(1H,3H)-二酮,1,7,8-三甲基-
羧甲基黄素
羟基-2-吡啶酮
维生素 B2
维他命 B2
硫酸氢3-(6,7-二氯-2,4-二羰基-3,4-二氢苯并[g]蝶啶-10(2H)-基)-N-乙基-N-(2-羟基乙基)丙烷-1-铵
硫酸氢2-(7,8-二氯-2,4-二羰基-3,4-二氢苯并[g]蝶啶-10(2H)-基)-N,N-二甲基乙铵
甲氨蝶呤钠
甲氨蝶呤杂质1
生物蝶呤-d3
生物喋呤中间体
环己烯,3-氟-4-(甲硫基)-,反-(9CI)
玫瑰黄色素
溴化氢溴化1-(2-氨基乙基)-3-甲基-4-[(Z)-2-萘-1-基乙烯基]吡啶正离子
氯化3-(7-氯-2,4-二羰基-3,4-二氢苯并[g]蝶啶-10(2H)-基)-N,N-二甲基丙烷-1-铵
氨蝶呤钠
氨苯蝶啶
氨甲酸,[(1S)-2-羟基-1-甲基丙基]-,1,1-二甲基乙基酯(9CI)
氨甲蝶呤
氨基蝶呤
核黄素还原
核黄素杂质Q
核黄素5'-硫酸盐
核黄素3′,4′-二磷酸酯
核黄素-4'-磷酸
核黄素-3'-磷酸盐
核黄素,2',3',4',5'-四乙酸酯
核黄素 5'-丁酸酯
核黄素
无色喋呤
异黄蝶呤
己二酸,2-[[4-[[(2-氨基-1,4,5,6,7,8-六氢-4-羰基-6-蝶啶基)甲基]氨基]苯甲酰]氨基]-
左亚叶酸钙杂质
左亚叶酸钙
四氢蝶酰五谷氨酸酯