(E)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(2-methoxyphenyl)prop-2-en-1-one | 118267-80-4
分子结构分类
中文名称
——
中文别名
——
英文名称
(E)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(2-methoxyphenyl)prop-2-en-1-one
英文别名
2'-Hydroxy-2,4',6'-trimethoxychalcone
CAS
118267-80-4
化学式
C18H18O5
mdl
——
分子量
314.338
InChiKey
KKTYCZKXENFEJP-CMDGGOBGSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:171-173 °C
-
沸点:529.9±50.0 °C(Predicted)
-
密度:1?+-.0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:23
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:65
-
氢给体数:1
-
氢受体数:5
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-羟基-4,6-二甲氧基苯乙酮 2-hydroxy-4,6-dimethoxyacetophenone 90-24-4 C10H12O4 196.203 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2',4,6-trimethoxyaurone 109309-38-8 C18H16O5 312.322
反应信息
-
作为反应物:描述:(E)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(2-methoxyphenyl)prop-2-en-1-one 在 吡啶 、 mercury(II) diacetate 作用下, 以68%的产率得到2',4,6-trimethoxyaurone参考文献:名称:Natural and synthetic 2′-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity摘要:A series of 2'-hydroxy-chalcones and their oxidative cyclization products, aurones, have been synthesized and tested for their antioxidant and lipoxygenase inhibitory activity. The natural product aureusidin (31) was synthesized in high yield by a new approach. An extensive structure-relationship study was performed and revealed that several chalcones and aurones possess an appealing pharmacological profile combining high antioxidant and lipid peroxidation activity with potent soybean LOX inhibition. (C) 2009 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2009.10.002
-
作为产物:描述:2-羟基-4,6-二甲氧基苯乙酮 、 邻甲氧基苯甲醛 在 sodium hydroxide 作用下, 以 乙醇 为溶剂, 反应 0.5h, 生成 (E)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(2-methoxyphenyl)prop-2-en-1-one参考文献:名称:通过Algar-Flynn-Oyamada(AFO)反应合成5-取代的黄酮醇:机理上的意义摘要:本文中,我们报告了一种合成方法,该方法通过使用碳酸钠/过氧化氢,通过Algar-Flynn-Oyamada反应(AFO)对5-取代的黄酮醇进行了改进,具有中等至高收率的一系列5-取代的黄酮醇。阐明了AFO反应的机理。LCMS分析和原位1 H NMR分析表明,在碱性碱/过氧化物条件下,环氧化物参与了从查尔酮到黄酮醇和/或金酮的转化。DOI:10.1016/j.tet.2017.06.064
文献信息
-
Design, Synthesis and Docking Studies of Flavokawain B Type Chalcones and Their Cytotoxic Effects on MCF-7 and MDA-MB-231 Cell Lines作者:Addila Abu Bakar、Muhammad Akhtar、Norlaily Mohd Ali、Swee Yeap、Ching Quah、Wan-Sin Loh、Noorjahan Alitheen、Seema Zareen、Zaheer Ul-Haq、Syed ShahDOI:10.3390/molecules23030616日期:——Flavokawain B (1) is a natural chalcone extracted from the roots of Piper methysticum, and has been proven to be a potential cytotoxic compound. Using the partial structure of flavokawain B (FKB), about 23 analogs have been synthesized. Among them, compounds 8, 13 and 23 were found in new FKB derivatives. All compounds were evaluated for their cytotoxic properties against two breast cancer cell linesFlavokawain B(1)是从Piper methysticum的根中提取的天然查尔酮,已被证明是潜在的细胞毒性化合物。使用黄酮类固醇B的部分结构(FKB),已经合成了约23个类似物。其中,在新的FKB衍生物中发现了化合物8、13和23。评估了所有化合物对两种乳腺癌细胞MCF-7和MDA-MB-231的细胞毒性,从而建立了结构-活性关系。FKB衍生物16(IC50 = 6.50±0.40和4.12±0.20μg/ mL),15(IC50 = 5.50±0.35和6.50±1.40μg/ mL)和13(IC50 = 7.12±0.80和4.04±0.30μg/ mL)对MCF-7和MDA-MB-231细胞系具有潜在的细胞毒性作用。但是,甲氧基在化合物2的第3位和第4位取代(IC50 = 8.90±0.60和6.80±0。35μg/ mL)和22(IC50 = 8.80±0.35和14.16±1
-
Synthesis of 2-Benzylidene-3(2<i>H</i>)-benzofuran-3-ones (Aurones) by Oxidation of 2′-Hydroxychalcones with Mercury(II) Acetate作者:Haruo SekizakiDOI:10.1246/bcsj.61.1407日期:1988.4The reaction of 2′-hydroxychalcones with mercury(II) acetate in acetic acid gives predominantly 2-benzylidene-3(2H)-benzofuran-3-ones (aurones) in 28–62% yield accompanied by flavanones in 5–21% yield.
-
人PCID2蛋白在制备或筛选抗肿瘤药物中的应用及具有抗肿瘤活性的化合物
-
Mechanisms of action and structure-activity relationships of cytotoxic flavokawain derivatives作者:Charlotte Thieury、Nicolas Lebouvier、Rémy Le Guével、Yann Barguil、Gaëtan Herbette、Cyril Antheaume、Edouard Hnawia、Yoshinori Asakawa、Mohammed Nour、Thierry GuillaudeuxDOI:10.1016/j.bmc.2017.01.049日期:2017.322 Flavokawain derivatives (FKd) were obtained by one step syntheses in order to conduct a SAR study to understand the structural requirements for optimum and selective cytotoxicity. FKd and natural flavokawains A and B found into kava, a South Pacific traditional beverage, were evaluated against nine cancer and one healthy cell lines. The targeted cell cycle phases as well as the effects on the induction of apoptosis and cell cycle protein levels were investigated. Therapeutic improvements (more activity and selectivity) were achieved with FKd compared to natural flavokawains and notably with the 2',3,4',6'-tetramethoxychalcone (FKd 19). FKd induced a Gl/S arrest on p53 wild-type cells and an M arrest on p53 mutant-type, via the up-regulation of p21 and cyclin B1 proteins, followed by apoptosis. Moreover, FKd exhibited a 24 h-effect on Akt/mTor normal cells versus a 48 h-effect on Akt/mTor up-regulated cells. The SAR study resulted in the conclusion that trimethoxy A-ring allowed the best compromise between cytotoxicity and selectivity, as well as the substitution of the meta position on the B-ring and the use of halogens substituents. (C) 2017 Elsevier Ltd. All rights reserved.
-
Efficient One‐Pot Synthesis of Hydroxyflavanones by Cyclization and<i>O</i>‐Demethylation of Methoxychalcones作者:Tao Liu、Huazhou Ying、Guan Lin、Yongzhou HuDOI:10.1080/00397910801991465日期:2008.5An efficient one-pot method for the synthesis of hydroxyflavanones is described. Methoxychalcones are treated with 36% HBr to afford cyclization and regioselective O-demethylation products (2a-i) while cyclization and complete O-demethylation products (3a-e) are obtained in the presence of 45% HI.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
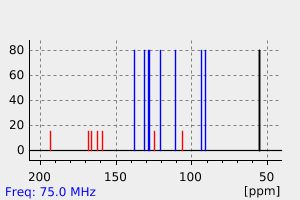
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2Z)-1,3-二苯基-2-丙烯-1-酮,2-丙烯-1-酮,1,3-二苯基-,(2Z)-
龙血素D
龙血素A
龙血素 B
黄色当归醇F
黄色当归醇B
黄腐醇; 黄腐酚
黄腐醇 D; 黄腐酚 D
黄腐酚B
黄腐酚
黄腐酚
黄卡瓦胡椒素 C
高紫柳查尔酮
阿普非农
阿司巴汀
阿伏苯宗
金鸡菊查耳酮
邻肉桂酰苯甲酸
达泊西汀杂质25
豆蔻明
补骨脂色烯查耳酮
补骨脂查耳酮
补骨脂呋喃查耳酮
补骨脂乙素
蜡菊亭; 4,2',4'-三羟基-6'-甲氧基查耳酮
苯酚,4-[3-(2-羟基苯基)-1-苯基丙基]-2-(3-苯基丙基)-
苯磺酰胺,N-[4-[3-(3-羟基苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,N-[3-[3-(4-羟基-3-甲氧苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,4-甲氧基-N,N-二甲基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯化,4,5-二甲氧基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯,4-甲氧基-3-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯甲醇,4-甲氧基-a-[2-(4-甲氧苯基)乙烯基]-
苯甲酸-[4-(3-氧代-3-苯基-丙烯基)-苯胺]
苯甲酸,3-[3-(4-溴苯基)-1-羰基-2-丙烯基]-4-羟基-
苯甲酰(2-羟基苯酰)甲烷
苯甲腈,4-(1-羟基-3-羰基-3-苯基丙基)-
苯基[2-(1-萘基)乙烯基]甲酮
苯基-(三苯基-丙-2-炔基)-醚
苯基-(2-苯基-2,3-二氢-苯并噻唑-2-基)-甲酮
苯亚甲基苯乙酮
苯乙酰腈,a-(1-氨基-2-苯基亚乙基)-
苯丙酸,a-苯甲酰-b-羰基-,苯基(苯基亚甲基)酰肼
苯,1-(2,2-二甲基-3-苯基丙基)-2-甲基-
苏木查耳酮
苄桂哌酯
苄基(4-氯-2-(3-氧代-1,3-二苯基丙基)苯基)氨基甲酸酯
芦荟提取物
腈苯唑
胀果甘草宁C
聚磷酸根皮酚