2-溴-2,3-二甲基丁烷 | 594-52-5
中文名称
2-溴-2,3-二甲基丁烷
中文别名
——
英文名称
2-bromo-2,3-dimethylbutane
英文别名
2-Brom-2,3-dimethyl-butan
CAS
594-52-5
化学式
C6H13Br
mdl
——
分子量
165.073
InChiKey
NILGDLGIGRGWRL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:25°C
-
沸点:147.26°C (estimate)
-
密度:1.1804
-
保留指数:896;903;921
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:7
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,3-二溴-2,3-二甲基丁烷 2,3-dibromo-2,3-dimethylbutane 594-81-0 C6H12Br2 243.969 1,2-二溴-2,3-二甲基丁烷 1,2-Dibrom-2,3-dimethyl-butan 29916-45-8 C6H12Br2 243.969
反应信息
-
作为反应物:描述:参考文献:名称:Couturier, Annales de Chimie (Cachan, France), 1892, vol. <6>26, p. 476摘要:DOI:
-
作为产物:描述:参考文献:名称:Highly Branched Alkylphosphorus Compounds. I. Synthesis of 2,3-Dimethylbutylphosphonyl Chloride1,2摘要:DOI:10.1021/jo01026a025
文献信息
-
A Mechanistic Study of Halogen Addition and Photoelimination from π-Conjugated Tellurophenes作者:Elisa I. Carrera、Anabel E. Lanterna、Alan J. Lough、Juan C. Scaiano、Dwight S. SeferosDOI:10.1021/jacs.5b11649日期:2016.3.2electron-withdrawing substituents have the highest photochemical quantum efficiencies in the presence of an alkene trap, with efficiencies of up to 42.4% for a pentafluorophenyl-functionalized tellurophene. The photoelimination reaction was studied in detail through bromine trapping experiments and laser flash photolysis, and a mechanism is proposed. The photoreaction, which occurs by release of bromine radicals, is使用可见光驱动反应性的能力对于许多化学学科都很重要,并且对可持续化学具有重要意义。识别光化学活性化合物和理解光化学机制对于开发用于合成和催化的有用材料非常重要。在这里,我们报告了一系列带有吸电子和给电子取代基的光敏二苯基碲酚化合物,它们通过炔偶联/闭环或钯催化的同芳基化化学合成。研究了这些化合物的氧化还原化学,涉及溴的氧化加成和光消除,这对于涉及 X2 的储能反应很重要。利用密度泛函理论研究了氧化加成反应机理,其结果支持三步机制,包括初始 η(1) 缔合复合物、单溴化中间体和最终二溴化产物的形成。根据化合物的吸收特性,所有碲酚衍生物都使用 430、447 或 617 nm 光进行光还原。在烯烃陷阱存在下,带有吸电子取代基的化合物具有最高的光化学量子效率,五氟苯基官能化碲酚的效率高达 42.4%。通过溴捕获实验和激光闪光光解对光消除反应进行了详细研究,并提出了机理。通过释放溴自由基发生的光反
-
The functionalization of saturated hydrocarbons. Part 23. Gif-type bromination and chlorination of saturated hydrocarbons: a non-radical reaction作者:Derek H.R. Barton、Éva Csuhai、Darío DollerDOI:10.1016/s0040-4020(01)85610-6日期:1992.1The bromination of saturated hydrocarbons was studied in the GoAggIII system using CBrCl3 and other polyhaloalkanes. This bromination reaction was compared to free radical processes by (i) evaluating the rates of reactions for a series of polyhaloalkanes, by (ii) measuring the selectivity of the different systems towards various saturated hydrocarbons and by (iii) analyzing the product distribution
-
Hydroalumination of alkenes by the LiAlH4 � 3AlBr3 system作者:E. V. Gorobets、O. V. Shitikova、S. I. Lomakina、G. A. Tolstikov、A. V. KuchinDOI:10.1007/bf00699198日期:1993.93AlBr3 system in low-polar solvents was studied. Alkenes with mono-, di-, tri-, and tetraalkyl substituted, mono- and diaryl substituted double bonds and anthracene react at room temperature to give the corresponding dibromoaluminoalkanes in high yields. Benzylidenefluorene, tetraphenylethylene, naphthalene, and phenanthrene do not undergo hydroalumination under these conditions. Camphene, bicyclo[3.2
-
A convenient and general preparation of alkyl hydroperoxides and dialkyl peroxides作者:Peter G. Cookson、Alwyn G. Davies、Brian P. RobertsDOI:10.1039/c39760001022日期:——Primary, secondary, and tertiary alkyl hydroperoxides and dialkyl peroxides can be prepared from the appropriate alkyl bromide or iodide and hydrogen peroxide or alkyl hydroperoxide in the presence of silver trifluoroacetate.
-
Boron trifluoride etherate/halide ion, a novel reagent for the conversion of allyl, benzyl and tertiary alcohols to the halides
表征谱图
-
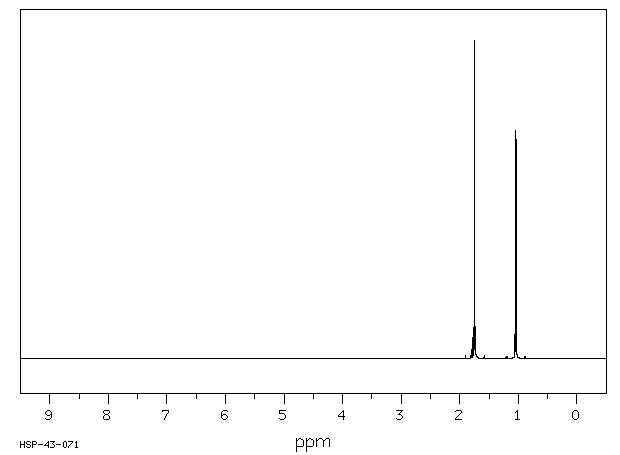
氢谱1HNMR
-
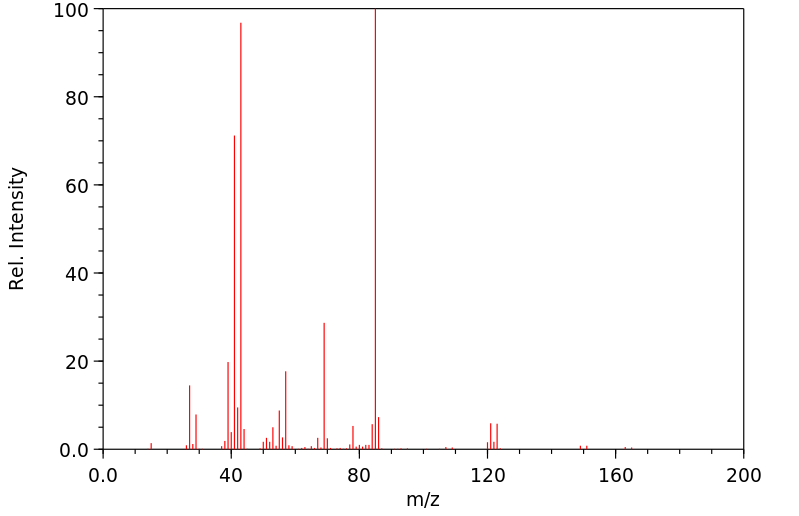
质谱MS
-
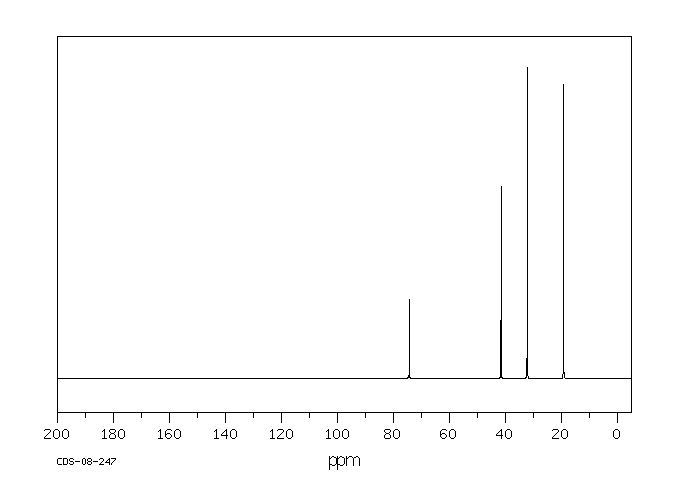
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-溴-1-丙炔-1-基)环丙烷
马杜拉霉素
顺-3,顺-6-1-溴壬二烯
顺,反,顺-1,2,3,4-四(2-溴乙基)环丁烷
金刚烷-2,2-d2
辛烷,1,5-二溴-
苯并噻唑,6-异硫氰酸根合5-甲基-(9CI)
苯(甲)醛,3-甲氧基-4-硝基-
硬脂基溴
硫杂二溴化
癸基溴
甲基环丙基溴化镁
环戊醇1-乙基-3-(苯甲基)-(9CI)
环戊烯-1,3-溴-(7CI,9CI)
环丙烷,1-溴-1-(3,3-二甲基-1-丁炔基)-2,2-二甲基-
环丁基溴
溴甲基环戊烷
溴甲基环己烷
溴甲基环丙烷
溴甲基环丁烷
溴甲基
溴环戊烷-D9
溴己烷-D3
溴己烷
溴化环辛基甲基
溴代环辛烷
溴代环戊烷
溴代环庚烷
溴代环丙烷
溴代异辛烷
溴代异丁烷
溴代叔丁烷-D9
溴代叔丁烷
溴代十四烷-D29
溴代十四烷
溴代十六烷-D33
溴代十六烷
溴代十五烷
溴代十二烷
溴代二十烷
溴乙醛
溴乙烷-D3
溴乙烷-D1
溴乙烷-2-13C
溴乙烷-13C2
溴乙烷-1-13C
溴乙烷-1,1-d2
溴乙烷-1,1,2,2-d4
溴乙烷
溴丙烷-D4