2-甲基-3-苯基苯甲醇 | 76350-90-8
中文名称
2-甲基-3-苯基苯甲醇
中文别名
2-甲基-3-羟甲基联苯;3-羟甲基-2-甲基联苯;2-甲基-(1,1'-联苯)-3-甲醇;联苯醇;2-甲基联苯-3-甲醇
英文名称
(2-methyl-[1,1'-biphenyl]-3-yl)methanol
英文别名
(2-methyl-[1,1′-biphenyl]-3-yl)methanol;3-hydroxymethyl-2-methylbiphenyl;(2-methyl-[1,1’-biphenyl]-3-yl)methanol;2-Methyl-3-biphenylmethanol;(2-methyl-3-phenylphenyl)methanol
CAS
76350-90-8
化学式
C14H14O
mdl
MFCD00134200
分子量
198.265
InChiKey
BGTLHJPGBIVQLJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:73-76 °C(lit.)
-
沸点:330.9±11.0 °C(Predicted)
-
密度:1.072±0.06 g/cm3(Predicted)
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
稳定性/保质期:
在指定条件下稳定,远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
WGK Germany:3
-
海关编码:2942000000
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
-
储存条件:存放在密封容器内,并置于阴凉、干燥处。
SDS
3-Hydroxymethyl-2-methylbiphenyl
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 3-Hydroxymethyl-2-methylbiphenyl
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 3-Hydroxymethyl-2-methylbiphenyl
Percent: >98.0%(GC)
CAS Number: 76350-90-8
Synonyms: 2-Methylbiphenyl-3-methanol
Chemical Formula: C14H14O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
3-Hydroxymethyl-2-methylbiphenyl
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Slightly pale reddish yellow
Odour: No data available
pH: No data available
Melting point/freezing point:72°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
3-Hydroxymethyl-2-methylbiphenyl
Section 10. STABILITY AND REACTIVITY
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
3-Hydroxymethyl-2-methylbiphenyl
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 3-Hydroxymethyl-2-methylbiphenyl
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 3-Hydroxymethyl-2-methylbiphenyl
Percent: >98.0%(GC)
CAS Number: 76350-90-8
Synonyms: 2-Methylbiphenyl-3-methanol
Chemical Formula: C14H14O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
3-Hydroxymethyl-2-methylbiphenyl
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Slightly pale reddish yellow
Odour: No data available
pH: No data available
Melting point/freezing point:72°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
3-Hydroxymethyl-2-methylbiphenyl
Section 10. STABILITY AND REACTIVITY
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
3-Hydroxymethyl-2-methylbiphenyl
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
应用
3-羟甲基-2-甲基联苯是由3-氯-2-甲基联苯与金属镁反应生成格氏试剂,再与聚甲醛二乙酸酯反应得到的产品,是杀虫剂联苯菊酯的中间体。
制备将(3-溴-2-甲基苯基)甲醇(4.1g, 5mmol)、苯基硼酸(5.0g, 10mmol)溶解在甲苯(12mL)和乙醇(4mL)中,搅拌后加入PdCl2(dppf).DCM(0.04g, 0.05mmol)及2M NaHCO3水溶液(4mL),用氮气脱气10分钟后,在80℃的密封管中加热12小时。通过硅藻土过滤反应混合物,滤液用水稀释,并用乙酸乙酯(2x100mL)萃取。有机层经硫酸钠干燥后蒸发,粗产物在Combiflash MPLC上进行纯化,以20%乙酸乙酯的己烷溶液作为洗脱液,得到灰白色固体3-羟甲基-2-甲基联苯(6, 产率:73%)。
化学性质该化合物为白色结晶状物质,熔点为73~76℃。它溶于乙醇、苯、甲苯等有机溶剂,而不溶于水。
用途2-甲基-(1,1′-联苯)-3-甲醇是生产杀虫剂联苯菊酯的中间体。
生产方法- 第一步:将3-氯-2-甲基联苯与四氢呋喃加入反应器中,再加入新鲜和回收的金属镁进行回流5小时后,加入聚甲醛二乙酸酯继续回流数小时。冷却后进行后处理、蒸馏得到产品,收率可达70.8%。
- 第二步:以3-氯-2-甲基联苯为原料,在氰化亚铜和吡啶的存在下反应生成3-氰基-2-甲基联苯,再还原成2-甲基-联苯-3-甲醛,进一步还原得2-甲基-(1,1′-联苯)-3-甲醇。
- 第三步:以2-甲基-3-硝基苯甲醇为原料,在氢气还原下生成2-甲基-3-氨基苯甲醇,然后在亚硝酸钠、碘化钾和铜粉的存在下进行Sander Mayer反应生成3-碘-2-甲基苯甲醇。接着在光反应器中经紫外灯照射与苯反应得到2-甲基(1,1′-联苯)-3-甲醇。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1,3-dihydro-4-phenylisobenzofuran 502483-86-5 C14H12O 196.249 2-甲基-[1,1'-联苯]-3-甲醛 2-methyl-[1,1'-biphenyl]-3-carbaldehyde 89951-60-0 C14H12O 196.249 1-(氯甲基)-2-甲基-3-苯基苯 3-chloromethyl-2-methyl-[1,1'-biphenyl] 84541-46-8 C14H13Cl 216.71 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-[1,1'-联苯]-3-羧酸 2-methyl-[1,1′-biphenyl]-3-carboxylic acid 115363-11-6 C14H12O2 212.248 —— (2-methyl-[1,1′-biphenyl]-3-yl)methanamine 1061650-37-0 C14H15N 197.28 —— 3-(bromomethyl)-2-methyl-1,1'-biphenyl —— C14H13Br 261.161 2-甲基-[1,1'-联苯]-3-甲醛 2-methyl-[1,1'-biphenyl]-3-carbaldehyde 89951-60-0 C14H12O 196.249 1-(氯甲基)-2-甲基-3-苯基苯 3-chloromethyl-2-methyl-[1,1'-biphenyl] 84541-46-8 C14H13Cl 216.71 —— biphenyl-2,3-dicarboxylic acid 4445-49-2 C14H10O4 242.231 —— 3-bromo-4-((2-methyl-[1,1'-biphenyl]-3-yl)methoxy)benzaldehyde —— C21H17BrO2 381.269 —— methyl 3-phenyl-2-[(trimethylsilyl)methyl]benzyl carbonate 935740-92-4 C19H24O3Si 328.483
反应信息
-
作为反应物:描述:2-甲基-3-苯基苯甲醇 在 4-二甲氨基吡啶 吡啶 、 正丁基锂 、 四甲基乙二胺 作用下, 以 四氢呋喃 、 正己烷 、 二氯甲烷 为溶剂, 反应 16.0h, 生成 methyl 3-phenyl-2-[(trimethylsilyl)methyl]benzyl carbonate参考文献:名称:Palladium-Catalyzed Formal [4+2] Cycloaddtion of o-Xylylenes with Olefins摘要:o-(Silylmethyl)benzylic carbonates reacted with various conjugated olefins in the presence of the palladium catalyst, which was generated in situ from Pd(eta(3)-C3H5)Cp and 1,2-bis(diphenylphosphino)ethane (DPPE). The reaction provided 2- and/or 3-substituted tetralins in good yield. The catalytic reaction described here is equivalent to the [4+2] cycloaddition of o-xylylenes with dienophiles.DOI:10.1021/ja070012l
-
作为产物:描述:参考文献:名称:Regiochemistry in the reductive opening of phthalan derivatives摘要:The lithiation of phthalan derivatives 4, 9 and 12 with an excess of lithium in the presence of a catalytic amount of 4,4'-ditert-butylbiphenyl (DTBB) in THF at -78 degrees C gives dianionic intermediates 5, 10 and 13, respectively, which by reaction with different electrophiles [H2O, tau-BuCHO, Me2CO, (EtO)(2)CO] at the same temperature, followed by hydrolysis, leads to regioselective functionalised naphthalenes 7, and biphenyls 11 and 14. The reductive opening takes place with high or total regioselectivity and can be explained considering the electron density in the dianion or in the radical anion, which are formed previous to the carbon-oxygen bond excision. The lithiation of the dihydrofurophthalan derivative 18 with the same reaction mixture but at higher temperature (0 degrees C) leads to intermediates 19 and 20, resulting from a single and a double reductive cleavage, respectively, which after addition of H2O and benzaldehyde as electrophiles gives a mixture of compounds 21 and 22. (c) 2007 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2007.03.069
文献信息
-
[EN] SUBSTITUTED 1,1'-BIPHENYL COMPOUNDS, ANALOGUES THEREOF, AND METHODS USING SAME<br/>[FR] COMPOSÉS 1,1'-BIPHÉNYLE SUBSTITUÉS, ANALOGUES DE CEUX-CI, ET PROCÉDÉS LES UTILISANT申请人:ARBUTUS BIOPHARMA INC公开号:WO2019191624A1公开(公告)日:2019-10-03The present invention includes substituted 3,3'-bis(phenoxymethyl)-1,1'-biphenyl compounds, analogues thereof, and compositions comprising the same, that can be used to treat or prevent hepatitis B virus (HBV) and/or hepatitis D virus (HDV) infections in a patient.本发明涵盖了替代的3,3'-双(苯氧甲基)-1,1'-联苯化合物,其类似物,以及包含它们的组合物,可用于治疗或预防患者体内的乙型肝炎病毒(HBV)和/或丙型肝炎病毒(HDV)感染。
-
An asymmetric Salamo-based Zn complex supported on Fe<sub>3</sub>O<sub>4</sub> MNPs: a novel heterogeneous nanocatalyst for the silyl protection and deprotection of alcohols under mild conditions作者:Hongyan Yao、Yongsheng Wang、Maryam Kargar RaziDOI:10.1039/d1ra01185e日期:——Fe3O4@H2L-Zn MNPs as a magnetically separable, recyclable and reusable heterogeneous catalyst. Fe3O4@H2L-Zn MNPs were also applied for the removal of silyl protecting groups from hydroxyl functions using water in CH2Cl2 under green conditions. The catalyst demonstrated good to excellent catalytic yield efficiency for both the reactions compared to the commercial metal-based catalysts under green conditions for在本研究中,磁性不对称 Salamo 基 Zn 配合物(H 2 L = salen 型二希夫碱)负载在改性 Fe 3 O 4 (Fe 3 O 4 @H 2 L-Zn) 的表面上作为一种新的磁性材料。催化剂通过多种分析技术进行设计和表征,例如 FT-IR 光谱、XRD、EDS、ICP-AES、SEM、TEM、TGA 和 VSM。提出了一种有效且可持续的合成方案,用于在温和条件下通过醇的甲硅烷基保护合成甲硅烷基醚子结构。该合成方案涉及各种含羟基底物和作为廉价甲硅烷基化剂的六甲基二硅氮烷(HMDS)之间的双组分无溶剂反应,使用Fe 3 O 4 @H 2 L-Zn MNP作为磁性可分离、可回收和可重复使用的多相催化剂。 Fe 3 O 4 @H 2 L-Zn MNP 也可用于在绿色条件下使用 CH 2 Cl 2中的水从羟基官能团中去除甲硅烷基保护基团。与商业金属基催化剂相比,该催化剂在绿色条件下对多种底物表现出良好至优异的催化产率。
-
Cross-linked poly(4-vinylpyridine/styrene) copolymer-supported bismuth(III) triflate: an efficient heterogeneous catalyst for silylation of alcohols and phenols with HMDS作者:Sang-Hyeup Lee、Santosh T. KadamDOI:10.1002/aoc.1809日期:2011.8Cross‐linked poly(4‐vinylpyridine/styrene) copolymer‐supported bismuth(III) triflate (30P/S‐Bi) effectively activates hexamethyldisilazane (HMDS) for the silylation of alcohols and phenols. By the use of this heterogeneous catalytic system, a wide range of alcohols as well as phenols are converted into their corresponding trimethylsilyl ethers in high yield under mild reaction conditions. The catalyst was reused
-
Method for producing cyclopropanecarboxylates申请人:——公开号:US20020052525A1公开(公告)日:2002-05-02There is provided a method for producing a cyclopropanecarboxylate of formula (1): 1 which comprises contacting a cyclopropanecarboxylate of formula (2): 2 with a monohydroxy compound of formula (3): R 7 OH (3) in the presence of a lithium compound of formula (4): R 8 OLi (4), wherein R 1 , R 2 , R 3 , R 4 and R 5 each independently represent a hydrogen atom, a halogen atom, a substituted or unsubstituted alkyl group, a substituted or unsubstituted alkenyl group, or a substituted or unsubstituted aryl group; R 6 represents an alkyl group having 1 to 10 carbon atoms or a substituted or unsubstituted phenyl group; R 7 and R 8 do not simultaneously represent the same and each independently represent a substituted or unsubstituted alkyl group, or a substituted or unsubstituted aryl group.
-
联苯类化合物及其制备方法和医药用途
表征谱图
-
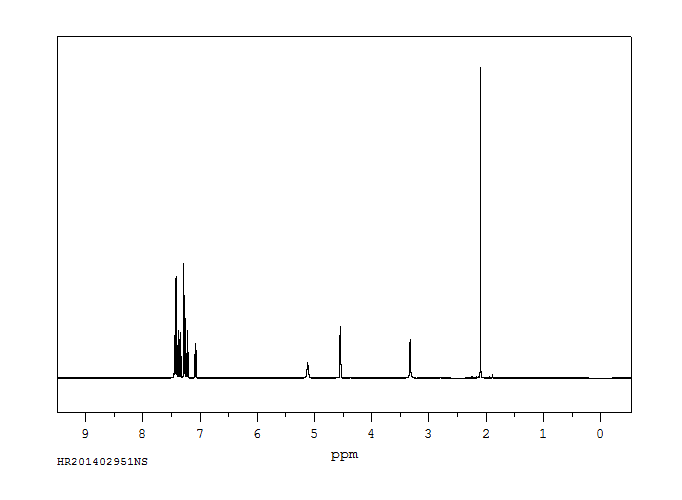
氢谱1HNMR
-
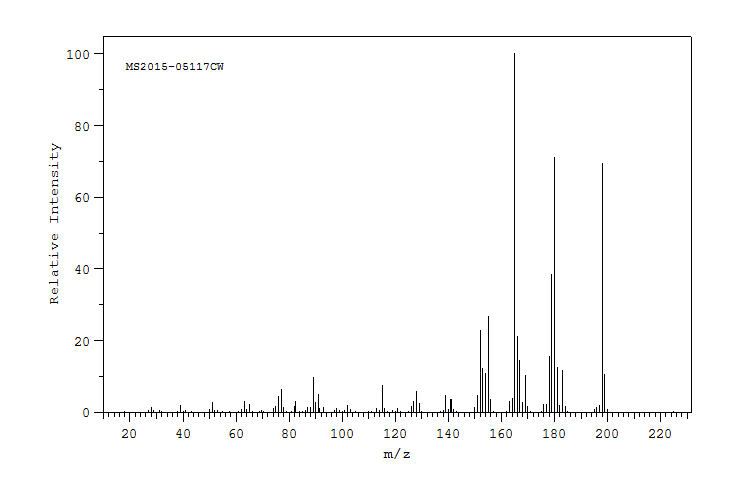
质谱MS
-
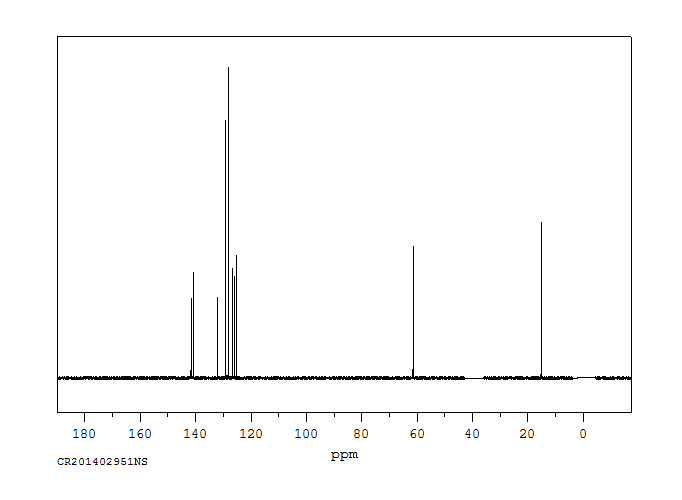
碳谱13CNMR
-
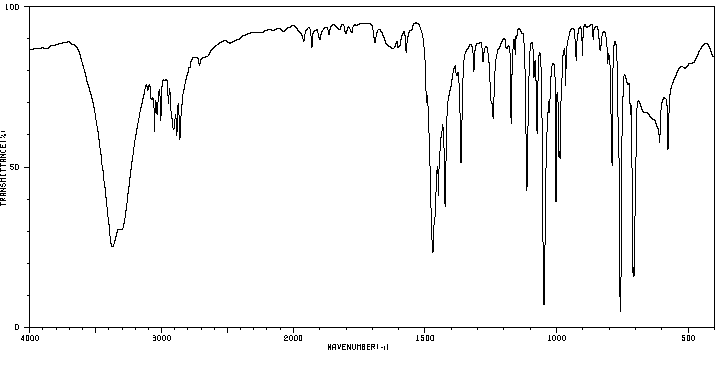
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫