2-hydroxy-4,4-dimethyl-5-oxo-N-phenylcyclohexenecarboxamide | 7721-66-6
中文名称
——
中文别名
——
英文名称
2-hydroxy-4,4-dimethyl-5-oxo-N-phenylcyclohexenecarboxamide
英文别名
2-(Phenylaminohydroxymethylene)-5,5-dimethylcyclohexane-1,3-dione;2-hydroxy-4,4-dimethyl-6-oxo-N-phenylcyclohexene-1-carboxamide
CAS
7721-66-6
化学式
C15H17NO3
mdl
——
分子量
259.305
InChiKey
SQDWNHRSJXSPSO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:19
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:66.4
-
氢给体数:2
-
氢受体数:3
反应信息
-
作为反应物:描述:2-hydroxy-4,4-dimethyl-5-oxo-N-phenylcyclohexenecarboxamide 在 草酰氯 、 三乙胺 、 N,N-二甲基甲酰胺 作用下, 以 二氯甲烷 、 乙腈 为溶剂, 反应 22.0h, 生成 2-(benzylthio)-4,4-dimethyl-6-oxo-N-phenylcyclohex-1-ene-1-carboxamide参考文献:名称:合成,结构-活性关系研究和作为趋化因子受体2(CXCR2)拮抗剂的3-氨基环己-2-烯-1-酮的ADMET性质摘要:在这里,我们描述了作为新型趋化因子受体2(CXCR2)拮抗剂的3-氨基环己-2-烯-1-酮衍生物的合成及其与构效关系。在针对CXCR2的探戈分析中,发现44种衍生物中的13种抑制CXCR2下游信号传导,IC 50值小于10μm。在计算机模拟中ADMET的预测表明,所有活性化合物都具有类似药物的特性。这些化合物均未显示出明显的细胞毒性,表明它们在炎症介导的疾病中的潜在应用。已经生成了结构-活性关系(SAR)图,以更好地了解它们的结合机制,以指导进一步优化这些新的CXCR2拮抗剂。DOI:10.1002/cmdc.201800027
-
作为产物:描述:3-羟基-5,5-二甲基环己-2-烯-1-酮 在 copper(l) iodide 、 氯化亚砜 、 硫酸 、 caesium carbonate 、 N,N-二甲基甲酰胺 作用下, 以 四氢呋喃 、 N,N-二甲基甲酰胺 为溶剂, 反应 63.0h, 生成 2-hydroxy-4,4-dimethyl-5-oxo-N-phenylcyclohexenecarboxamide参考文献:名称:一种利用二氧化碳生产杀虫剂的工艺方法摘要:一种利用二氧化碳生产杀虫剂的工艺方法,步骤如下:称取1,3‑环己二酮类底物、催化剂和Cs2CO3于Schleck瓶中,脱气,持续通入1atm的二氧化碳。加入溶剂,在50℃油浴中,反应48h。反应完成后,后处理,得到2(a‑e)。将得到的酸酰化,然后滴加到含有苯胺的二氯甲烷溶液中,常温下反应2h。反应完后,柱层析分离,得到3(a‑e)。将3(a‑e)加入到50%的浓硫酸中,80℃回流8h。通过分离,得到4(a‑e)。本发明的优点是:催化剂制备简单,催化剂有高的催化活性,可回收利用,实现工业循环,达到可持续生产的目标;3(a‑e)和4(a‑e)的制备工艺简单,对设备要求较低,原料来源广泛且成本低,毒性低,易于工业化放大生产。公开号:CN109452280B
文献信息
-
Triphenylbismuthonio-4,4-dimethyl-2,6-dioxocyclohexane-1-ide and Triphenylbismuthonio-4,4-dimethyl-2,6-dioxo-3,5-dioxan-1-ide. Preparation and Properties of the Stable Bismuthonium Ylides作者:Hitomi Suzuki、Toshihiro Murafuji、Takuji OgawaDOI:10.1246/cl.1988.847日期:1988.5.5The title two bismuthonium ylides were obtained in pure form and their reactions with some electrophiles were studied. In contrast with stable ylides of arsenic and antimony, these bismuthonium ylides readily react with aldehydes and isothiocyanates but they are inert towards ketones.
-
Enols of Carboxylic Acid Amides with β-Electron-Withdrawing Substituents作者:Jayanta Kumar Mukhopadhyaya、Stepan Sklenák、Zvi RappoportDOI:10.1021/ja992059f日期:2000.2.1The effect of stabilizing enols of carboxamides by several two β-electron-withdrawing substituents was studied with the R1R2CHCONHPh systems. When R1R2CH2 = Meldrum's acid (MA), the solid-state structure is that of the enol R1R2CC(OH)NHPh (7). In CDCl3 solution the structure is 7, but there may be some exchange on the NMR time scale with a tautomer. B3LYP/6-31G** calculations show a significant preference用 R1R2CHCONHPh 系统研究了几个两个 β-吸电子取代基稳定羧酰胺的烯醇的效果。当 R1R2CH2 = Meldrum 酸 (MA) 时,固态结构是烯醇 R1R2CC(OH)NHPh (7)。在CDCl3 溶液中,结构为7,但在NMR 时间尺度上可能存在一些与互变异构体的交换。B3LYP/6-31G** 计算表明,烯醇 R1R2CC(OH)NH2 (12a)(R1R2C = MA 部分)和 (MeO2C)2CC(OH)NHPh (11b) 比酰胺结构更偏爱烯醇结构。然而,固体 11 具有酰胺结构 (MeO2C)2CHCONHPh (11a)。CDCl3 中的 NMR 谱显示 >90% 的 11a,但也存在少量物质,可能是 11b。在 DMSO 中没有观察到这种物质。类似的二甲酮取代的苯胺 10 以固态和溶液形式作为环羰基的烯醇存在。计算表明,HC(CO2Me)3 的能量低于其互变
-
Pharmaceutical use of substituted amides申请人:Andersen Sune Henrik公开号:US20060111366A1公开(公告)日:2006-05-25The use of substituted amides for modulating the activity of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) and the use of these compounds as pharmaceutical compositions, are described. Also a novel class of substituted amides, their use in therapy, pharmaceutical compositions comprising the compounds, as well as their use in the manufacture of medicaments are described. The present compounds are modulators and more specifically inhibitors of the activity of 11βHSD1 and may be useful in the treatment, prevention and/or prophylaxis of a range of medical disorders where a decreased intracellular concentration of active glucocorticoid is desirable.
-
Amide Derivatives and Pharmaceutical Use Thereof申请人:Andersen Henrik Sune公开号:US20090264414A1公开(公告)日:2009-10-22The use of substituted amides for modulating the activity of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) and the use of these compounds as pharmaceutical compositions, are described. Also a novel class of substituted amides, their use in therapy, pharmaceutical compositions comprising the compounds, as well as their use in the manufacture of medicaments are described. The present compounds are modulators and more specifically inhibitors of the activity of 11βHSD1 and may be useful in the treatment, prevention and/or prophylaxis of a range of medical disorders where a decreased intracellular concentration of active glucocorticoid is desirable.
-
Combinations of an 11-beta-hydroxysteroid dehaydrogenase type 1 inhibitor and a glucocorticoid receptor agonist申请人:NOVO NORDISK A/S公开号:EP1854487A2公开(公告)日:2007-11-14Combination therapy comprising the administration of an 11β-hydroxysteroid dehydrogenase type 1 inhibitor and a glucocorticoid receptor agonist for treating some forms of cancer, diseases and disorders having inflammation as a component, and to minimize the side effects associated with glucorticoid receptor agonist therapy.
表征谱图
-
氢谱1HNMR
-
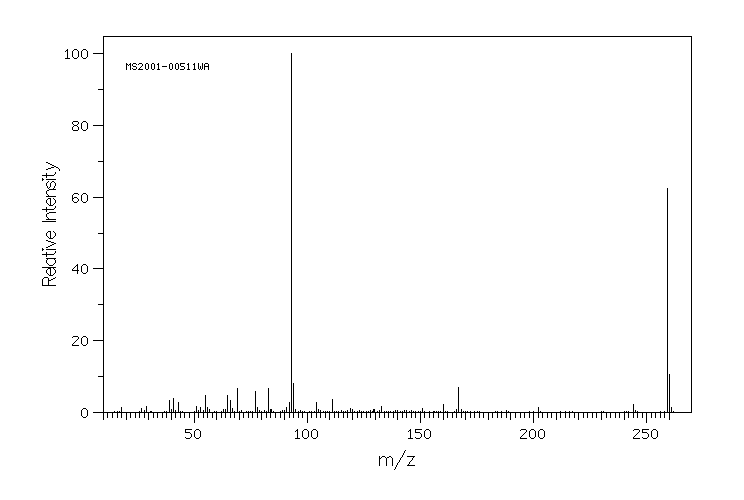
质谱MS
-
碳谱13CNMR
-
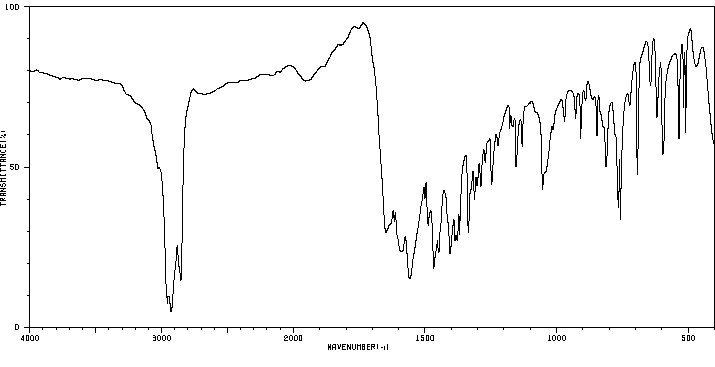
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫