甲基-异丙基丙二酸二乙酯 | 58447-69-1
中文名称
甲基-异丙基丙二酸二乙酯
中文别名
——
英文名称
diethyl 2-isopropyl-2-methylmalonate
英文别名
Diethyl isopropylmethylmalonate;diethyl 2-methyl-2-propan-2-ylpropanedioate
CAS
58447-69-1
化学式
C11H20O4
mdl
——
分子量
216.277
InChiKey
NQSVPQIOJRYMGQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:15
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.82
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 异丙基丙二酸二乙酯 diethyl isopropylmalonate 759-36-4 C10H18O4 202.251 甲基丙二酸二乙酯 Diethyl methylmalonate 609-08-5 C8H14O4 174.197 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-异丙基-2-甲基丙二酸 isopropyl-methyl-malonic acid 75847-97-1 C7H12O4 160.17
反应信息
-
作为反应物:描述:甲基-异丙基丙二酸二乙酯 在 lithium aluminium tetrahydride 、 氯化亚砜 作用下, 以 吡啶 为溶剂, 反应 2.0h, 生成 2,3-methylbutanol tosylate参考文献:名称:Highly Branched Alkylphosphorus Compounds. I. Synthesis of 2,3-Dimethylbutylphosphonyl Chloride1,2摘要:DOI:10.1021/jo01026a025
-
作为产物:描述:参考文献:名称:氢与碲氢化钠轻松置换叔硝基摘要:碲化氢钠是一种用氢取代一类叔硝基的有用试剂。DOI:10.1246/bcsj.58.1067
文献信息
-
Catalytic reductive desymmetrization of malonic esters作者:Pengwei Xu、Zhongxing HuangDOI:10.1038/s41557-021-00715-0日期:2021.7carbons with a pair of enantiotopic functional groups is a practical strategy for the synthesis of quaternary stereocentres, as it divides the tasks of enantioselection and C−C bond formation. The use of disubstituted malonic esters as the substrate of desymmetrization is particularly attractive, given their easy and modular preparation, as well as the high synthetic values of the chiral monoester products
-
Synthese von 4,4-disubstituierten 1,3-Thiazol-5(4H)-thionen
-
Substituent effects on partition coefficients of barbituric acids作者:Ooi Wong、Robert Henry McKeownDOI:10.1002/jps.2600771105日期:1988.11Precise partition coefficients in 1-octanol-water at 25 degrees C were determined for three 2-thiobarbituric acids and 14 barbituric acids with a wider range of substituents. The experimental log P values (log Pexp) of barbituric acids were correlated with the carbon number and the branching effect of the C5 substituent(s) by linear regression analysis. The carbon number term makes a major contribution
-
光稳定剂化合物及包含其的液晶组合物申请人:株式会社东进世美肯公开号:CN110835317A公开(公告)日:2020-02-25
-
Desymmetric Partial Reduction of Malonic Esters作者:Pengwei Xu、Shihao Liu、Zhongxing HuangDOI:10.1021/jacs.2c01380日期:2022.4.20malonic esters is a rewarding strategy to access structurally diverse quaternary stereocenters. Particularly, asymmetric reduction of malonic esters would generate a functional group with a lower oxidation state than the remaining ester, thus allowing for more chemoselective derivatization. Here, we report a new set of conditions for the zinc-catalyzed desymmetric hydrosilylation of malonic esters that容易获得的二取代丙二酸酯的去对称化是一种获得结构多样的季立体中心的有益策略。特别是,丙二酸酯的不对称还原将产生一个氧化态低于剩余酯的官能团,从而允许更多的化学选择性衍生化。在这里,我们报告了一组锌催化的丙二酸酯不对称氢化硅烷化的新条件,这些条件提供了醛作为主要产物。与醇选择性去对称化相比,部分还原使用更高浓度的硅烷和新的哌啶醇衍生的四齿配体,建议将半缩醛锌中间体的途径从消除转换为甲硅烷基化。因此,从具有大量取代基的丙二酸酯中获得了高醛醇比和醛的对映选择性。连同醛的丰富反应性,部分还原使得能够快速合成含有四元立体中心的生物活性化合物和天然代谢物。,容易获得的二取代丙二酸酯的去对称化是一种获得结构多样的季立体中心的有益策略。特别是,丙二酸酯的不对称还原将产生一个氧化态低于剩余酯的官能团,从而允许更多的化学选择性衍生化。在这里,我们报告了一组锌催化的丙二酸酯不对称氢化硅烷化的新条件,这些条件提供
表征谱图
-
氢谱1HNMR
-
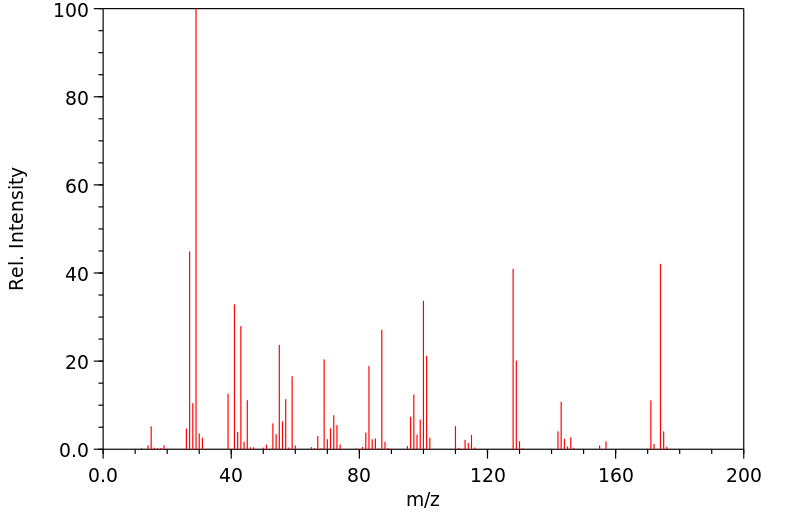
质谱MS
-
碳谱13CNMR
-
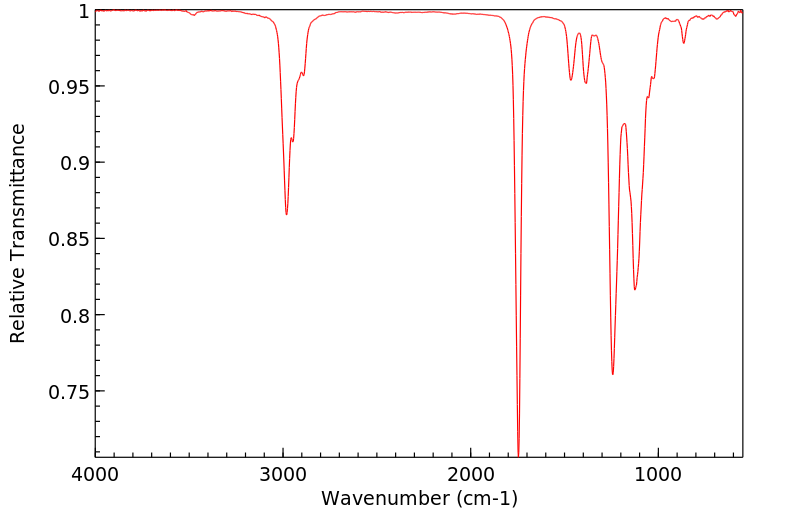
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯