3,3,6-三甲基-1,2-二氢茚 | 40650-41-7
中文名称
3,3,6-三甲基-1,2-二氢茚
中文别名
——
英文名称
1,1,5-trimethyl-2,3-dihydro-1H-indene
英文别名
1,1,5-trimethylindan;1,1,5-Trimethyl-indan;2,3-dihydro-1,1,5-trimethyl-1H-indene;1,1,5-trimethylindane;3,3,6-trimethyl-1,2-dihydroindene
CAS
40650-41-7
化学式
C12H16
mdl
——
分子量
160.259
InChiKey
JIHZHLCOASGNCG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:246.18°C (rough estimate)
-
密度:0.9119
-
保留指数:1217.2
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,3,6-三甲基 -1-茚酮 3,3,6-Trimethyl-1-indanone 54484-71-8 C12H14O 174.243
反应信息
-
作为反应物:描述:3,3,6-三甲基-1,2-二氢茚 在 硝酸 作用下, 以29%的产率得到2,3-Dihydro-1,1,5-trimethyl-4,6-dinitro-1H-indene参考文献:名称:Synthesis and Herbicidal Activity of Substituted Tetrahydronaphthalenes (Part II)摘要:This paper reports the synthesis and the biological activity of novel tetrahydronaphthalenes with substitution of functional groups at each position of the aromatic ring and substitution of various alkyl groups at the 1-position of the non-aromatic ring. These compounds exhibited pre-emergent herbicidal activity which was determined by the orientation and type of functional groups on the aromatic ring with the 1,1-dimethyl substitution on the non-aromatic ring. The activity tended to be highest for nitro or methyl at the 5- and 7-positions with an amino or ester group at the 6-position and a dimethyl substitution at the 1-position.DOI:10.1002/(sici)1096-9063(199603)46:3<227::aid-ps358>3.0.co;2-v
-
作为产物:描述:7-(2-(tert-butyl)-5-methylphenyl)cyclohepta-1,3,5-triene 在 1,3‐bis[2,6‐bis(propan‐2‐yl)phenyl]‐2‐[(phenylformonitrile)aurio]‐2,3‐dihydro‐1H‐imidazol‐2‐yl hexafluorostibane 作用下, 以 1,2-二氯乙烷 为溶剂, 反应 7.0h, 以90%的产率得到3,3,6-三甲基-1,2-二氢茚参考文献:名称:通过环庚三烯的脱碳作用金(I)-催化的分子内C(sp3)-H插入摘要:基于分子内插入由逆布氏反应(脱碳)生成的金(I)碳烯的C(sp 3)-H键的新颖合成茚满和二氢萘烷的方法。氘标记和动力学同位素效应实验,DFT计算以及从特征明确的金(I)类胡萝卜素生成拟议的卡宾中间体均支持C(sp 3)-H功能化过程涉及三中心协同机制。DOI:10.1002/chem.201900919
文献信息
-
Odeur et constitution XVIII. Sur un dérivé indanique à odeur musquée作者:Cl. Ferrero、R. HelgDOI:10.1002/hlca.19590420640日期:——(1) The present paper describes the synthesis of 1,1-dimethyl-4-acetyl-6-t-butyl-indan, and particularly our structure investigation of this very strong musk compound discovered independently by M. G. J. Beets and co-workers and by the authors.
-
[EN] NOVEL OCTAHYDROINDENYL PROPANAL COMPOUNDS<br/>[FR] NOUVEAUX COMPOSÉS D'OCTAHYDRO-INDÈNYLE PROPANAL申请人:INT FLAVORS & FRAGRANCES INC公开号:WO2017066214A1公开(公告)日:2017-04-20The present invention pertains to novel octahydro-indene compounds and their unexpected advantageous use thereof in enhancing, improving or modifying the fragrance of perfumes, colognes, toilet waters, fabric care products, personal products, and the like.
-
Non-peptide GnRH agents, pharmaceutical compositions, and methods for their use申请人:Pfizer Inc.公开号:US20040053951A1公开(公告)日:2004-03-18Non-peptide GnRH agents that inhibit the effect of gonadotropin-releasing hormone are described. Such agents are useful for treating mammalian reproductive disorders and steroid hormone-dependent tumors as well as for regulating fertility, where suppression of gonadotropin release is indicated.
-
Non-peptide GnRH agents, Pharmaceutical compositions, and methods for their use申请人:Pfizer, Inc.公开号:US06833372B2公开(公告)日:2004-12-21Non-peptide GnRH agents that inhibit the effect of gonadotropin-releasing hormone are described. Such agents are useful for treating mammalian reproductive disorders and steroid hormone-dependent tumors as well as for regulating fertility, where suppression of gonadotropin release is indicated.
-
Non-peptide compounds affecting the action of gonadotropin-releasing hormone (GnRH)申请人:PFIZER INC.公开号:EP1334972B1公开(公告)日:2006-04-19
表征谱图
-
氢谱1HNMR
-
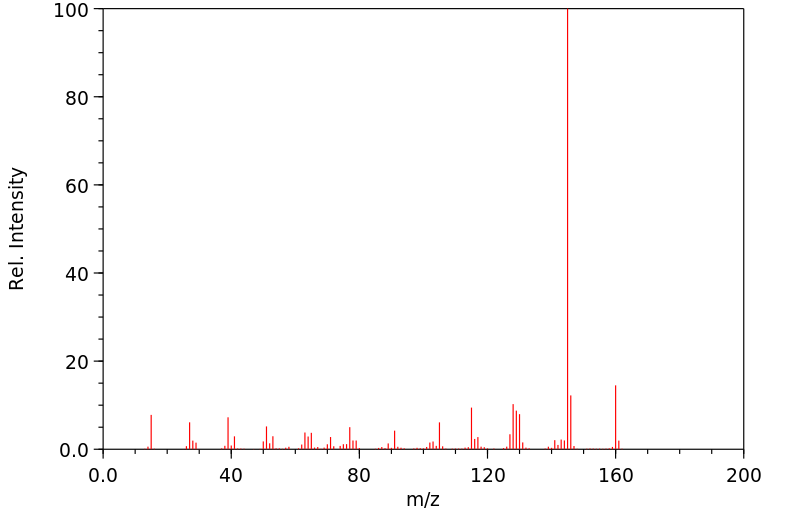
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(R)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(4S,5R)-3,3a,8,8a-四氢茚并[1,2-d]-1,2,3-氧杂噻唑-2,2-二氧化物-3-羧酸叔丁酯
(3aS,8aR)-2-(吡啶-2-基)-8,8a-二氢-3aH-茚并[1,2-d]恶唑
(3aS,3''aS,8aR,8''aR)-2,2''-环戊二烯双[3a,8a-二氢-8H-茚并[1,2-d]恶唑]
(1α,1'R,4β)-4-甲氧基-5''-甲基-6'-[5-(1-丙炔基-1)-3-吡啶基]双螺[环己烷-1,2'-[2H]indene
齐洛那平
鼠完
麝香
风铃醇
颜料黄138
顺式-1,6-二甲基-3-(4-甲基苯基)茚满
雷美替胺杂质9
雷美替胺杂质24
雷美替胺杂质14
雷美替胺杂质13
雷美替胺杂质10
雷美替胺杂质
雷美替胺杂质
雷美替胺杂质
雷美替胺杂质
雷美替胺杂质
雷美替胺
雷沙吉兰相关化合物HCl
雷沙吉兰杂质8
雷沙吉兰杂质5
雷沙吉兰杂质4
雷沙吉兰杂质3
雷沙吉兰杂质16
雷沙吉兰杂质15
雷沙吉兰杂质12
雷沙吉兰杂质1
雷沙吉兰杂质
雷沙吉兰13C3盐酸盐
雷沙吉兰
阿替美唑盐酸盐
铵2-(1,3-二氧代-2,3-二氢-1H-茚-2-基)-8-甲基-6-喹啉磺酸酯
金粉蕨辛
金粉蕨亭
重氮正癸烷
酸性黄3[CI47005]
酒石酸雷沙吉兰
还原茚三酮(二水)
还原茚三酮
过氧化,2,3-二氢-1H-茚-1-基1,1-二甲基乙基
贝沙罗汀杂质8
表蕨素L
螺双茚满
螺[茚-2,4-哌啶]-1(3H)-酮盐酸盐
螺[茚-2,4'-哌啶]-1(3H)-酮