3,4-二甲基-1,2,5-噁二唑 | 4975-21-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-7°C
-
沸点:183.6°C (rough estimate)
-
密度:1.0528
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:38.9
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
海关编码:2934999090
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:存储条件:2-8℃,干燥,密封。
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-(氯甲基)-4-甲基-1,2,5-恶二唑 4-chloromethyl-3-methylfurazan 62642-47-1 C4H5ClN2O 132.549 3-(溴甲基)-4-甲基-呋咱 3-(bromomethyl)-4-methyl-1,2,5-oxadiazole 90507-32-7 C4H5BrN2O 177.0 3,4-双(氯甲基)-1,2,5-噁二唑 3,4-bis(Chlormethyl)furazan 53601-88-0 C4H4Cl2N2O 166.995
反应信息
-
作为反应物:描述:参考文献:名称:[EN] 4(SPIROPIPERIDINYL)METHYL SUBSTITUTED PYRROLIDINES AS MODULATORS OF CHEMOKINE RECEPTOR ACTIVITY
[FR] 4(SPIROPIPERIDINYL)METHYL PYRROLIDINES SUBSTITUEES SERVANT DE MODULATEURS DE L'ACTIVITE DES RECEPTEURS DES CHIMIOKINES摘要:在环的4位上具有螺环哌啶甲基取代基的3-取代吡咯烷可用作化学因子受体活性调节剂。具体来说,这些化合物可用作化学因子受体CCR-3和/或CCR-5的调节剂。公开号:WO2004058763A1 -
作为产物:描述:参考文献:名称:有关类固醇的研究。IV。雄甾烷[2,3-c]呋喃丹及其相关化合物的合成。摘要:合成了与2, 3-位融合的呋噁环类固醇。通过2-羟基亚氨基-3-酮(IV)或2, 3-二酮(II)从雄甾-3-酮(I)制备的2, 3-二羟基亚氨基雄甾烷(III),在碱、琥珀酸酸酐或氯化亚硫酰的作用下,直接环化生成雄甾烷[2, 3-c]呋噁烷(XVII)。或者,将2, 3-二羟基亚氨基化合物(III)用次氯酸钠或四乙酸铅处理,得到相应的呋噁烷N-氧化物(呋氧烷)(XXI),再通过与三乙基磷酸酯加热处理去氧化为呋噁烷(XVII)。同样,合成了雄甾-4-烯和雄甾-4, 6-二烯[2, 3-c]呋噁烷(XVII, XIX)。还描述了其衍生物的合成。DOI:10.1248/cpb.13.1445
文献信息
-
Lithiation of five-membered heteroaromatic compounds. The methyl substituted 1,2-azoles, oxadiazoles, and thiadiazoles作者:R. G. MicetichDOI:10.1139/v70-334日期:1970.7.1
The lithiation of various methyl substituted isoxazoles, isothiazoles, pyrazoles, oxadiazoles, and thiadiazoles using n-butyllithium has been studied. Three types of reactions, namely, lateral lithiation, ring cleavage, and addition of butyllithium to the ring, have been found. 3,5-Dimethylisoxazole, 3-phenyl-5-methylisoxazole, 3,4-dimethyl-1,2,5-oxadiazole, 2,5-dimethyl-1,3,4-thiadiazole, 3-phenyl-5-methyl-1,2,4-oxadiazole, and 3,5-dimethyl-1,2,4-thiadiazole all undergo lateral lithiation to give the respective acetic acids after carboxylation. 1-Methyl-3,5-disubstituted pyrazoles form the 1-lithiomethyl derivatives, while 1-phenyl-3,5-disubstituted pyrazoles are converted to the 1-ortholithiophenyl-3,5-disubstituted pyrazoles. 4-Methylisothiazole is lithiated mainly at C-5, but also suffers ring cleavage to form 1-n-butylthio-2-cyanoprop-1-ene. Heteroaromatic compounds containing an N—S bond, such as 3,4-dimeth yl-1,2,5-thiadiazole, 4-methyl-5-phenyl-1,2,3-thiadiazole, and 3,5-dimethylisothiazole, undergo nucleophilic attack at sulfur with resulting ring cleavage. 3,5-Dimethylisothiazole produces 2-n-butylthiopent-2-en-4-one. 3-Methyl-5-phenyl-1,2,4-oxadiazole gave 3-methyl-5-phenyl-5-n-butyl-1,2,4-dihydroöxadiazole by addition to the azomethine bond. The results of these lithiations are discussed. 3-Methyl-5-lithiomethylisoxazole was converted to various derivatives. Nuclear magnetic resonance spectral analysis was used to establish the identity of the products.
各种甲基取代异噁唑、异硫唑、吡唑、噁二唑和噻二唑的锂化反应已经被研究。发现了三种类型的反应,即侧链锂化、环裂解和丁基锂加入环中。3,5-二甲基异噁唑、3-苯基-5-甲基异噁唑、3,4-二甲基-1,2,5-噁二唑、2,5-二甲基-1,3,4-噻二唑、3-苯基-5-甲基-1,2,4-噁二唑和3,5-二甲基-1,2,4-噻二唑都经历侧链锂化,在羧化后生成相应的乙酸。1-甲基-3,5-二取代吡唑形成1-锂甲基衍生物,而1-苯基-3,5-二取代吡唑转化为1-邻锂苯基-3,5-二取代吡唑。4-甲基异硫唑主要在C-5处发生锂化,但也发生环裂解形成1-正丁硫基-2-氰基丙-1-烯。含有N—S键的杂环化合物,如3,4-二甲基-1,2,5-噻二唑、4-甲基-5-苯基-1,2,3-噻二唑和3,5-二甲基异硫唑,在硫原子上发生亲核攻击,导致环裂解。3,5-二甲基异硫唑产生2-正丁硫代戊-2-烯-4-酮。3-甲基-5-苯基-1,2,4-噁二唑通过加成到偶氮亚胺键生成3-甲基-5-苯基-5-正丁基-1,2,4-二氢噁二唑。讨论了这些锂化的结果。3-甲基-5-锂甲基异噁唑被转化为各种衍生物。核磁共振光谱分析用于确定产物的身份。 -
Nonquaternary cholinesterase reactivators. 2. .alpha.-Heteroaromatic aldoximes and thiohydroximates as reactivators of ethyl methylphosphonyl-acetylcholinesterase in vitro作者:Richard A. Kenley、Clifford D. Bedford、Oliver D. Dailey、Robert A. Howd、Alexi MillerDOI:10.1021/jm00375a021日期:1984.9alpha-heteroaromatic aldoximes, RC(= NOH)H, and thiohydroximates, RC(= NOH)S-(CH2)2N(C2H5)2, where R represents various oxadiazole and thiadiazole rings. Each compound was characterized with respect to the following: structure, (hydroxyimino)methyl acid dissociation constant, nucleophilicity toward trigonal carbon and tetrahedral phosphorus, octanol-buffer partition coefficient, reversible inhibition of eel acetylcholinesterase我们准备了六对α-杂芳族醛肟(RC(= NOH)H和硫代氢肟酸酯RC(= NOH)S-(CH2)2N(C2H5)2,其中R代表各种恶二唑和噻二唑环。每种化合物的特征如下:结构,(羟基亚氨基)甲基解离常数,对三角碳和四面体磷的亲核性,辛醇-缓冲液分配系数,鳗e乙酰胆碱酯酶(AChE)的可逆性抑制以及被抑制的AChE的体外再活化由对硝基苯基甲基膦酸酯制得。十二种化合物中的八种可显着活化乙基甲基膦酰基-AChE,但固有反应性中等至低:最有效的非季铵化活化剂,3-苯基-5-[(羟基亚氨基)甲基] -1,2,4-恶二唑,与众所周知的活化剂2-[((羟基亚氨基)甲基] -1-甲基碘化碘化物(2-PAM))相比,它的活性低17倍。非季铵化合物之一,即3-苯基-1,2,4-恶二唑-5-硫代氢氧酸2-(二乙氨基)乙基S-酯,是AChE的强大可逆抑制剂(I50 = 7.5 microM)。非季化合物结构,活化
-
Morpholine derivatives, compositions containing them and their use as申请人:Merck Sharp & Dohme Ltd.公开号:US05877316A1公开(公告)日:1999-03-02The present invention relates to compounds of formula (I), wherein R.sup.1 and R.sup.4 represent hydrogen, halogen, C.sub.1-6 alkyl, C.sub.2-6 alkenyl, C.sub.2-6 alkynyl, C.sub.3-7 cycloalkyl, C.sub.3-7 cycloalkylC.sub.1-4 alkyl, C.sub.1-6 alkoxy, C.sub.1-4 alkyl substituted by a hydroxy or C.sub.1-4 alkoxy group, OCF.sub.3, hydroxy, trifluoromethyl, trimethylsilyl, nitro, CN, SR.sup.a, SOR.sup.a, SO.sub.2 R.sup.a, COR.sup.a, CO.sub.2 R.sup.a or CONR.sup.a R.sup.b, where R.sup.a and R.sup.b are each independently hydrogen or C.sub.1-4 alkyl; R.sup.2, R.sup.3 and R.sup.5 represent hydrogen, halogen, C.sub.1-6 alkyl, C.sub.1-4 alkoxy substituted by a C.sub.1-4 alkoxy group, or trifluoromethyl; R.sup.6 represents C.sub.1-6 alkyl, optionally substituted by oxo, substituted by a 5-membered heteroaromatic ring selected from oxazole, thiazole, isoxazole, isothiazole, oxadiazole and thiadiazole, wherein each heteroaromatic ring is substituted at the available carbon atom by a group of the formula: ZNR.sup.7 R.sup.8. The compounds are of particular use in the treatment of pain, inflammation, migraine and emesis.本发明涉及式(I)的化合物,其中R.sup.1和R.sup.4代表氢,卤素,C.sub.1-6烷基,C.sub.2-6烯基,C.sub.2-6炔基,C.sub.3-7环烷基,C.sub.3-7环烷基C.sub.1-4烷基,C.sub.1-6烷氧基,C.sub.1-4烷基被羟基或C.sub.1-4烷氧基取代,OCF.sub.3,羟基,三氟甲基,三甲基硅基,硝基,CN,SR.sup.a,SOR.sup.a,SO.sub.2R.sup.a,COR.sup.a,CO.sub.2R.sup.a或CONR.sup.aR.sup.b,其中R.sup.a和R.sup.b各自独立地表示氢或C.sub.1-4烷基;R.sup.2,R.sup.3和R.sup.5代表氢,卤素,C.sub.1-6烷基,C.sub.1-4烷氧基被C.sub.1-4烷氧基取代,或三氟甲基;R.sup.6代表C.sub.1-6烷基,可选择地被氧代取代,被选自噁唑,噻唑,异噁唑,异噻唑,噁二唑和噻二唑的5-成员杂环芳基环取代,其中每个杂环芳基环在可用的碳原子上被式ZNR.sup.7R.sup.8的基团取代。这些化合物在治疗疼痛,炎症,偏头痛和呕吐方面具有特殊用途。
-
Substituted pyridines from isoxazoles: scope and mechanism作者:Seokjoo Lee、Rashmi Jena、Aaron L. OdomDOI:10.1039/d2ob00779g日期:——Treatment of isoxazoles with enamines leads to an inverse electron-demand hetero-Diels–Alder reaction that produces substituted pyridines in the presence of TiCl4(THF)2 and titanium powder. The reaction is highly regioselective with only a single isomer of the product observed by GC/MS and tolerant of many common functional groups. The transformation was examined computationally, and it was found that
-
Organometallic synthesis in the furazan series. 4. Reactions of azofurazans with organolithium compounds作者:A. B. Sheremetev、E. A. Ivanova、E. V. Shatunova、D. E. Dmitriev、N. E. Kuz"minaDOI:10.1023/b:rucb.0000035645.16537.ea日期:2004.3The reactions of azofurazans, including macrocyclic azofurazans, with BunLi and Li derivatives of methylfurazans were studied. Several competitive processes were found to occur: the addition of a Li reagent at the N=N bond, the redox reaction giving rise to hydrazofurazans, and the reaction of the side chain of azofurazan.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
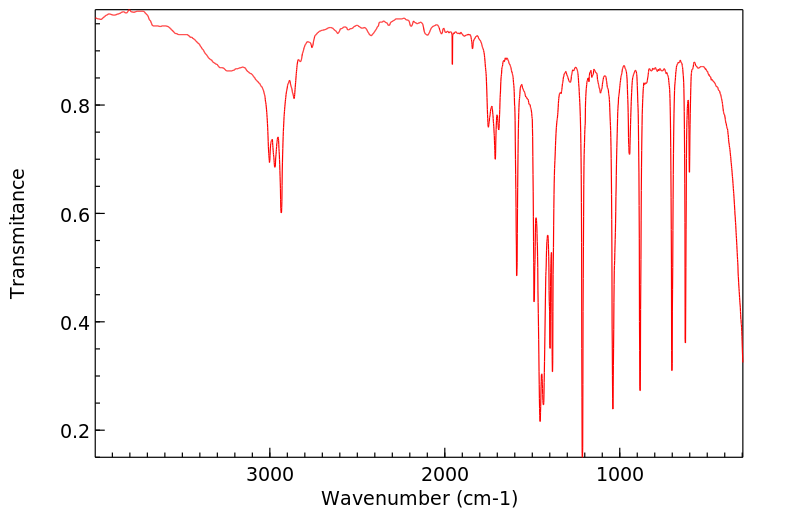
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息