甲基二氯环己基硅烷 | 5578-42-7
中文名称
甲基二氯环己基硅烷
中文别名
二氯环己基甲基硅烷;环己基甲基二氯硅烷;甲基环己基二氯硅烷
英文名称
dichlorocyclohexylmethylsilane
英文别名
cyclohexylmethyldichlorosilane;Methylcyclohexyldichlorosilane;dichloro-cyclohexyl-methylsilane
CAS
5578-42-7
化学式
C7H14Cl2Si
mdl
MFCD00046333
分子量
197.18
InChiKey
YUYHCACQLHNZLS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:<0°C
-
沸点:83 °C15 mm Hg(lit.)
-
密度:1.094 g/mL at 20 °C(lit.)
-
闪点:66°C
-
LogP:1.9 at 20℃
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险等级:8
-
危险品标志:C
-
安全说明:S26,S36/37/39,S45
-
危险类别码:R34
-
WGK Germany:3
-
海关编码:29310099
-
危险品运输编号:UN 2987 8/PG 2
-
储存条件:避光、阴凉干燥处,密封保存。
SDS
二氯环己基甲基硅烷 修改号码:5
模块 1. 化学品
产品名称: Dichlorocyclohexylmethylsilane
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
金属腐蚀性 第1级
健康危害
皮肤腐蚀/刺激 1B类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 可燃液体
可能腐蚀金属
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 远离明火/热表面。
只可存放于原用的容器内。
切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
吸收溢出物,防止材料被损坏。
二氯环己基甲基硅烷 修改号码:5
模块 2. 危险性概述
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 二氯环己基甲基硅烷
百分比: >95.0%(GC)
CAS编码: 5578-42-7
分子式: C7H14Cl2Si
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,二氧化碳
不适用的灭火剂: 水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 切勿与水接触。移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防
爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
可能产生高压。小心打开。
使用耐腐蚀设备。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
二氯环己基甲基硅烷 修改号码:5
模块 7. 操作处置与储存
存放于惰性气体环境中。
防湿。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
潮敏
包装材料: 依据法律。只可存放在原用的容器內。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 202 °C
闪点: 66°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.09
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 与水接触分解并产生有毒气体。
避免接触的条件: 明火, 湿气
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢, 氧化硅
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
二氯环己基甲基硅烷 修改号码:5
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
UN编号: 2987
正式运输名称: 氯硅烷, 腐蚀的, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Dichlorocyclohexylmethylsilane
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
金属腐蚀性 第1级
健康危害
皮肤腐蚀/刺激 1B类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 可燃液体
可能腐蚀金属
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 远离明火/热表面。
只可存放于原用的容器内。
切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
吸收溢出物,防止材料被损坏。
二氯环己基甲基硅烷 修改号码:5
模块 2. 危险性概述
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 二氯环己基甲基硅烷
百分比: >95.0%(GC)
CAS编码: 5578-42-7
分子式: C7H14Cl2Si
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,二氧化碳
不适用的灭火剂: 水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 切勿与水接触。移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防
爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
可能产生高压。小心打开。
使用耐腐蚀设备。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
二氯环己基甲基硅烷 修改号码:5
模块 7. 操作处置与储存
存放于惰性气体环境中。
防湿。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
潮敏
包装材料: 依据法律。只可存放在原用的容器內。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 202 °C
闪点: 66°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.09
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 与水接触分解并产生有毒气体。
避免接触的条件: 明火, 湿气
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢, 氧化硅
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
二氯环己基甲基硅烷 修改号码:5
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
UN编号: 2987
正式运输名称: 氯硅烷, 腐蚀的, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 环己基二甲基氯硅烷 chloro-cyclohexyl-dimethylsilane 71864-47-6 C8H17ClSi 176.761
反应信息
-
作为反应物:描述:参考文献:名称:在水-氯仿界面上具有金纳米颗粒组装的二有机硅烷催化氧化为1,1,3,3-四有机二硅氧烷†摘要:通过使用环四硅氧烷[RSCH 2 CH 2 SiMeO] 4(R = CH 2 CH 2 OH)作为水,在水-氯仿界面上形成200±20 nm尺寸的金纳米颗粒(AuNPs)的球形自组装体。稳定配体。界面稳定的AuNPs可作为多功能催化剂,用于选择性水解氧化仲有机硅烷中的一个Si–H键RR 1 SiH 2(R,R 1 =烷基,芳基和硅烷基),以提供较高的合成1,1,3,3-四有机二硅氧烷((HRR 1 Si)2O.这项研究首次揭示了在可见光辐射下激发AuNPs的表面等离振子共振所产生的光热效应在增强环境温度下的催化活性中的作用。DOI:10.1039/c8nj04223c
-
作为产物:参考文献:名称:甲硅烷基氢化:一种战略方法,可以直接使用通用的甲硅烷基饱和饱和碳和杂环化合物摘要:我们报告了一种通过芳烃加氢将易得的甲硅烷基化芳烃转化为甲硅烷基化饱和碳和杂环的方法。范围包括烷氧基和卤代甲硅烷基取代基。甲硅烷基可被衍生为多种功能,并在有机合成,材料科学以及制药,农业化学和香料研究中得到应用。但是,目前的技术很难获得甲硅烷基化的饱和(杂)循环。氢化的产率取决于硅胶添加剂的量。这种二氧化硅效应还可以显着改善以前公开的高度氟化芳烃的氢化方法(例如,氢化至全顺式C 6 H 6 F 6)。DOI:10.1002/anie.201804124
文献信息
-
Catalytic Synthesis of Functional Silicon-Stereogenic Silanes through<i>Candida antarctica</i>Lipase B Catalyzed Remote Desymmetrization of Silicon-Centered Diols作者:Xing Lu、Li Li、Wei Yang、Kezhi Jiang、Ke-Fang Yang、Zhan-Jiang Zheng、Li-Wen XuDOI:10.1002/ejoc.201300932日期:2013.9A series of silicon-containing diols are synthesized and used in lipase-catalyzed remote desymmetrization. This synthetic method is valuable in the construction of optically active silicon-stereogenic organosilicon compounds. Good enantioselectivities of the remote desymmetrization was achieved with Candida antarctica lipase B (CAL-B) (up to 90:10er).
-
Synthesis of Cyclic Peroxides Containing the Si-<i>gem</i>-bisperoxide Fragment. 1,2,4,5,7,8-Hexaoxa-3-silonanes as a New Class of Peroxides作者:Alexander O. Terent'ev、Maxim M. Platonov、Anna I. Tursina、Vladimir V. Chernyshev、Gennady I. NikishinDOI:10.1021/jo7027213日期:2008.4.1A method was developed for the synthesis of the previously unknown class of organic peroxides, 1,2,4,5,7,8-hexaoxa-3-silonanes, based on the reaction of dialkyldichlorosilanes with 1,1‘-dihydroperoxyperoxides. 1,2,4,5,7,8-Hexaoxa-3-silonanes are rather stable under ambient conditions and were characterized by NMR spectroscopy, X-ray diffraction, and elemental analysis. Their yields are in a range of
-
A cyclic manipulation of cage isomers <i>via</i> anion exchange and thermal isomerism作者:Seonghyeon Park、Dongwon Kim、Doheon Kim、Dongwook Kim、Ok-Sang JungDOI:10.1039/d0cc08303h日期:——A cyclic manipulation of peanut cage isomers has been achieved via anion exchange and unusual cage isomerism.通过阴离子交换和异常的笼形异构性已经实现了花生笼形异构体的循环操作。
-
Construction of Axial Chirality and Silicon-Stereogenic Center via Rh-Catalyzed Asymmetric Ring-Opening of Silafluorenes作者:Xiufen Bi、Jia Feng、Xiaoping Xue、Zhenhua GuDOI:10.1021/acs.orglett.1c00935日期:2021.4.16A rhodium-catalyzed enantioselective ring-opening/acylation of silafluorenes is reported. The newly developed bulky phosphoramidite ligand, in combination with methanol as the additive, enabled the reaction to create one axial chirality and one silicon-stereogenic center in a highly selective manner by only cleavage of one Si–C bond.
-
Hydrosilylation of cyclohexene, 1-methylcyclohexene, and isopropylidenecyclohexane作者:O. G. Yarosh、L. V. Zhilitskaya、N. K. Yarosh、A. I. Albanov、M. G. VoronkovDOI:10.1007/s11176-005-0114-4日期:2004.12Hydrosilylation of cyclohexene and isopropylidenecyclohexane with chloro(methyl)silanes Me3−n SiHCln (n = 1–3) gives rise to cyclohexyl- and chloro(2-cyclohexylpropyl)methylsilanes. Hydrosilylation of 1-methylcyclohexene with chlorodimethylsilane (n = 1) occurs anomalously and involves double-bond migration to form a mixture of seven compounds: the cis and trans isomers of 2-, 3-, 4-chlorodimethyl(methylcyclohexyl)silanes and chlorodimethyl(cyclohexylmethyl)silane. Chlorodimethylsilane (n = 2) adds to 1-methylcyclohexene to form a mixture of the cis and trans isomers of dichloro(methyl)(2-methylcyclohexyl)silane and dichloro(cyclohexylmethyl)methylsilane. With trichlorosilane (n = 3), no other products than trichloro(cyclohexylmethyl)silane are formed. The hydrosilylation products were reacted with ethynylmagnesium bromide to synthesize the corresponding ethynyl derivatives.
表征谱图
-
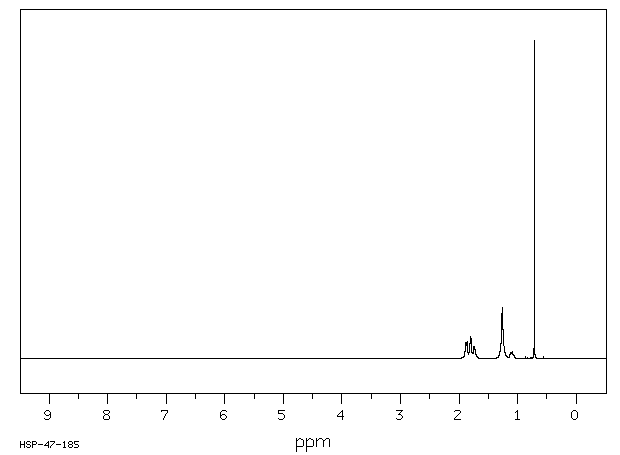
氢谱1HNMR
-
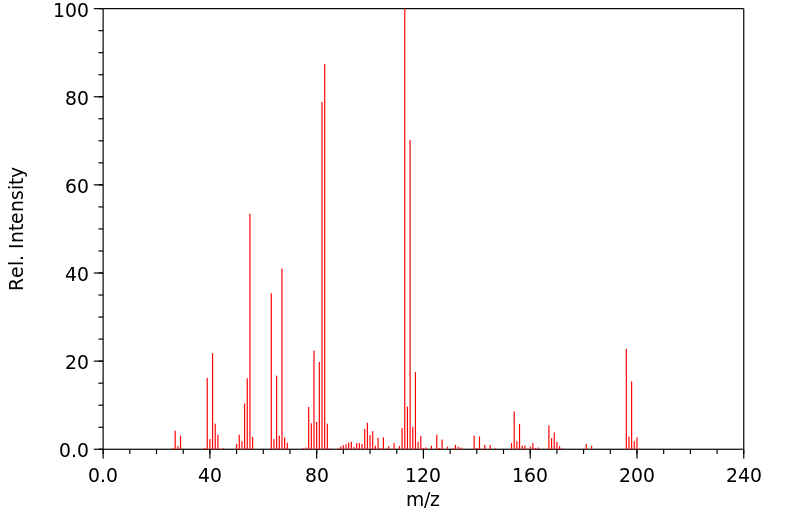
质谱MS
-
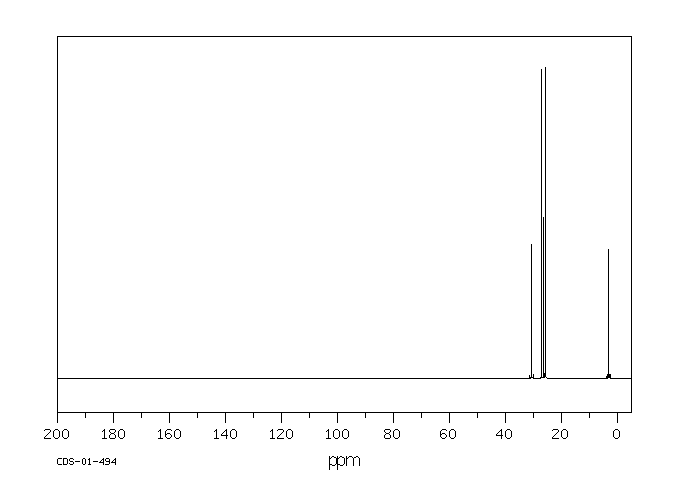
碳谱13CNMR
-
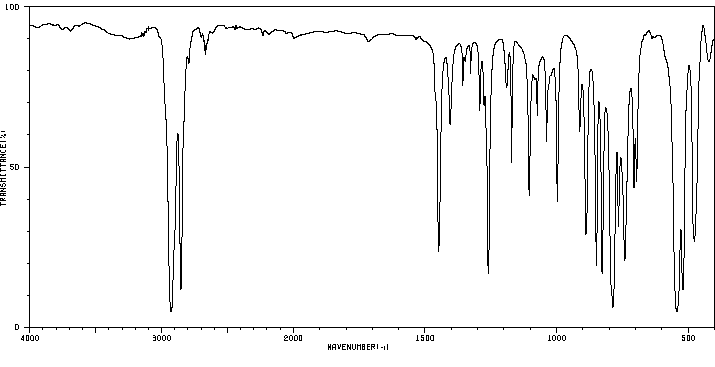
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-溴乙氧基)-特丁基二甲基硅烷
鲸蜡基聚二甲基硅氧烷
骨化醇杂质DCP
马沙骨化醇中间体
马来酸双(三甲硅烷)酯
顺式-二氯二(二甲基硒醚)铂(II)
顺-N-(1-(2-乙氧基乙基)-3-甲基-4-哌啶基)-N-苯基苯酰胺
降钙素杂质13
降冰片烯基乙基三甲氧基硅烷
降冰片烯基乙基-POSS
间-氨基苯基三甲氧基硅烷
镓,二(1,1-二甲基乙基)甲基-
镁,氯[[二甲基(1-甲基乙氧基)甲硅烷基]甲基]-
锑,二溴三丁基-
铷,[三(三甲基甲硅烷基)甲基]-
铂(0)-1,3-二乙烯-1,1,3,3-四甲基二硅氧烷
钾(4-{[二甲基(2-甲基-2-丙基)硅烷基]氧基}-1-丁炔-1-基)(三氟)硼酸酯(1-)
金刚烷基乙基三氯硅烷
酰氧基丙基双封头
达格列净杂质
辛醛,8-[[(1,1-二甲基乙基)二甲基甲硅烷基]氧代]-
辛甲基-1,4-二氧杂-2,3,5,6-四硅杂环己烷
辛基铵甲烷砷酸盐
辛基衍生化硅胶(C8)ZORBAX?LP100/40C8
辛基硅三醇
辛基甲基二乙氧基硅烷
辛基三甲氧基硅烷
辛基三氯硅烷
辛基(三苯基)硅烷
辛乙基三硅氧烷
路易氏剂-3
路易氏剂-2
路易士剂
试剂Cyanomethyl[3-(trimethoxysilyl)propyl]trithiocarbonate
试剂3-[Tris(trimethylsiloxy)silyl]propylvinylcarbamate
试剂3-(Trimethoxysilyl)propylvinylcarbamate
试剂2-(Trimethylsilyl)cyclopent-2-en-1-one
试剂11-Azidoundecyltriethoxysilane
西甲硅油杂质14
衣康酸二(三甲基硅基)酯
苯胺,4-[2-(三乙氧基甲硅烷基)乙基]-
苯磺酸,羟基-,盐,单钠聚合甲醛,1,3,5-三嗪-2,4,6-三胺和脲
苯甲醇,a-[(三苯代甲硅烷基)甲基]-
苯并磷杂硅杂英,5,10-二氢-10,10-二甲基-5-苯基-
苯基二甲基氯硅烷
苯基二甲基乙氧基硅
苯基二甲基(2'-甲氧基乙氧基)硅烷
苯基乙酰氧基三甲基硅烷
苯基三辛基硅烷
苯基三甲氧基硅烷