3-氟磺酰基苯甲酸 | 454-95-5
中文名称
3-氟磺酰基苯甲酸
中文别名
——
英文名称
3-carboxybenzenesulfonyl fluoride
英文别名
3-(fluorosulfonyl)benzoic acid;4-(fluorosulfonyl)benzoic acid;3-fluorosulfonyl benzoic acid;3-fluorosulfonyl-benzoic acid;3-Fluorsulfonyl-benzoesaeure;Benzoesaeure-sulfofluorid-(3);3-fluorosulfonylbenzoic acid
CAS
454-95-5
化学式
C7H5FO4S
mdl
MFCD00007415
分子量
204.179
InChiKey
VWYMBWGOJRULOV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:147-153°C
-
沸点:358.8±25.0 °C(Predicted)
-
密度:1.538±0.06 g/cm3(Predicted)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:79.8
-
氢给体数:1
-
氢受体数:5
安全信息
-
危险等级:8
-
海关编码:2916399090
-
包装等级:III
-
危险类别:8
-
WGK Germany:3
-
危险品运输编号:UN 1759
-
危险性防范说明:P260,P280,P303+P361+P353,P301+P330+P331,P304+P340+P310,P305+P351+P338+P310
-
危险性描述:H314
-
储存条件:应存于室温、密封、干燥且惰性气体环境中。
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : 3-Fluorosulfonyl-Benzoic Acid
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 454-95-5
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Eye irritation (Category 2), H319
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Xi Irritant R36
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Warning
Hazard statement(s)
H319 Causes serious eye irritation.
Precautionary statement(s)
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
Other hazards
This substance/mixture contains no components considered to be either persistent, bioaccumulative and
toxic (PBT), or very persistent and very bioaccumulative (vPvB) at levels of 0.1% or higher.
SECTION 3: Composition/information on ingredients
Substances
Formula : C7H5FO4S
Molecular weight : 204,18 g/mol
CAS-No. : 454-95-5
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
3-Fluorosulfonyl-Benzoic Acid
Eye Irrit. 2; H319 <= 100 %
Hazardous ingredients according to Directive 1999/45/EC
Component Classification Concentration
3-Fluorosulfonyl-Benzoic Acid
Xi, R36 <= 100 %
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
No data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
No data available
Advice for firefighters
Wear self-contained breathing apparatus for firefighting if necessary.
Further information
No data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure
adequate ventilation. Avoid breathing dust.
For personal protection see section 8.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Handle and store under inert gas.
Storage class (TRGS 510): Non Combustible Solids
Specific end use(s)
Apart from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges.
Use respirators and components tested and approved under appropriate government standards
such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour No data available
c) Odour Threshold No data available
d) pH No data available
e) Melting point/freezing No data available
point
f) Initial boiling point and No data available
boiling range
g) Flash point No data available
h) Evaporation rate No data available
i) Flammability (solid, gas) No data available
j) Upper/lower No data available
flammability or
explosive limits
k) Vapour pressure No data available
l) Vapour density No data available
m) Relative density No data available
n) Water solubility No data available
o) Partition coefficient: n- No data available
octanol/water
p) Auto-ignition No data available
temperature
q) Decomposition No data available
temperature
r) Viscosity No data available
s) Explosive properties No data available
t) Oxidizing properties No data available
Other safety information
No data available
SECTION 10: Stability and reactivity
Reactivity
No data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
No data available
Conditions to avoid
No data available
Incompatible materials
No data available
Hazardous decomposition products
Other decomposition products - No data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
No data available
Skin corrosion/irritation
No data available
Serious eye damage/eye irritation
No data available
Respiratory or skin sensitisation
No data available
Germ cell mutagenicity
No data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
No data available
Specific target organ toxicity - single exposure
No data available
Specific target organ toxicity - repeated exposure
No data available
Aspiration hazard
No data available
Additional Information
RTECS: Not available
SECTION 12: Ecological information
Toxicity
No data available
Persistence and degradability
No data available
Bioaccumulative potential
No data available
Mobility in soil
No data available
Results of PBT and vPvB assessment
This substance/mixture contains no components considered to be either persistent, bioaccumulative and
toxic (PBT), or very persistent and very bioaccumulative (vPvB) at levels of 0.1% or higher.
Other adverse effects
No data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
No data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
No data available
Chemical Safety Assessment
For this product a chemical safety assessment was not carried out
SECTION 16: Other information
Full text of H-Statements referred to under sections 2 and 3.
Eye Irrit. Eye irritation
H319 Causes serious eye irritation.
Full text of R-phrases referred to under sections 2 and 3
Xi Irritant
R36 Irritating to eyes.
Further information
Copyright 2014 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-氯磺酰基苯甲酸 3-Carboxybenzenesulfonyl chloride 4025-64-3 C7H5ClO4S 220.633 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 3-(fluorosulfonyl)benzoate 124397-36-0 C8H7FO4S 218.206 —— 3-(hydroxymethyl)benzenesulfonyl fluoride 96892-89-6 C7H7FO3S 190.195 —— 3-carbamoyl-benzenesulfonyl fluoride 454-94-4 C7H6FNO3S 203.194 3-(氟磺酰基)苯甲酰氯 3-fluorosulfonyl-benzoyl chloride 454-93-3 C7H4ClFO3S 222.624 3-氯磺酰基苯甲酸 3-Carboxybenzenesulfonyl chloride 4025-64-3 C7H5ClO4S 220.633 3-[(3-羧基苯基)磺酰基]-1,2-三氮杂二烯-2-鎓-1-I去 3-carboxybenzenesulfonyl azide 15980-11-7 C7H5N3O4S 227.2
反应信息
-
作为反应物:描述:3-氟磺酰基苯甲酸 在 dimethyl sulfide borane 作用下, 以 四氢呋喃 为溶剂, 以100%的产率得到3-(hydroxymethyl)benzenesulfonyl fluoride参考文献:名称:霍纳尔-沃兹沃思-埃蒙斯的方式与有效的抗癌活性piperlongumine类似物†摘要:具有抗癌活性的天然产物在铅和靶标的发现中起着至关重要的作用。我们在这里报告了植物来源的生物碱,哌隆胺和类似物的合成和生物学评估。使用Horner-Wadsworth-Emmons偶联方法,可以从新型膦酰乙酰胺试剂中以较高的总收率制备出类似哌隆胺的化合物。许多化合物通过可能涉及ROS产生的作用机理,对结直肠癌(HCT 116)和卵巢癌(IGROV-1)癌细胞系显示出有效的抗癌活性。与以前的报道相反,未观察到哌隆明类似物在癌细胞(MRC-5)中的选择性作用。DOI:10.1039/c6ob01160h
-
作为产物:参考文献:名称:芳香族磺酰氟共价动力学稳定运甲状腺素蛋白以防止淀粉样蛋白形成,同时提供荧光缀合物摘要:选择性地结合给定蛋白质,然后进行快速化学选择性反应以形成共价缀合物的分子在药物开发中具有实用性。本文设想了一个 1,3,4-恶二唑库,其 2 位被芳基磺酰氟取代,5 位被取代的芳基取代,已知该芳基对转甲状腺素蛋白 (TTR) 的内部甲状腺素结合亚位点具有高亲和力基于结构的设计原理并通过化学合成。当结合在甲状腺素结合位点时,大多数芳基磺酰氟会与 pKa 扰动的 K15 残基发生快速且化学选择性的反应,从而在动力学上稳定 TTR,从而防止已知会导致多发性神经病的淀粉样原纤维形成。结合 t50 范围为 1 至 4 分钟,比甲状腺素结合位点外的水解反应快约 1400 倍。X 射线晶体学证实了预期的结合方向,并揭示了磺酰氟活化导致磺酰胺与 TTR 的连接。一些芳基磺酰氟在血浆中与 TTR 有效形成缀合物。合成的 11 种 TTR 共价动力学稳定剂在缀合时表现出荧光,因此由于发色团的环境敏感荧光而可能具有成像应用。DOI:10.1021/ja311729d
文献信息
-
[EN] 6,7-DIHYDROPYRAZOLO[1,5-A]PYRAZIN-4(5H)-ONE COMPOUNDS AND THEIR USE AS NEGATIVE ALLOSTERIC MODULATORS OF MGLUR2 RECEPTORS<br/>[FR] COMPOSÉS 6,7-DIHYDROPYRAZOLO[1,5-A] PYRAZIN-4(5H)-ONE ET LEUR UTILISATION COMME MODULATEURS ALLOSTÉRIQUES NÉGATIFS DES RÉCEPTEURS MGLUR2申请人:JANSSEN PHARMACEUTICA NV公开号:WO2016016395A1公开(公告)日:2016-02-04The present invention relates to novel 6,7-dihydropyrazolo[1,5-a]pyrazin-4(5H)-one derivatives as negative allosteric modulators (NAMs) of the metabotropic glutamate receptor subtype 2 ("mGluR2"). The invention is also directed to pharmaceutical compositions comprising such compounds, to processes for preparing such compounds and compositions, and to the use of such compounds and compositions for the prevention or treatment of disorders in which the mGluR2 subtype of metabotropic receptors is involved.
-
Correlation of carbon-13 substituent-induced chemical shifts:Meta- andpara-substituted methyl benzoates作者:Miloš Buděšńský、Otto ExnerDOI:10.1002/mrc.1260270612日期:1989.6Carbon‐13 NMR spectra are reported for 69 substituted methyl benzoates in deuteriochloroform or in its mixture with dimethyl sulphoxide‐d6. The substituent‐induced chemical shifts (SCS) of the CO carbon correlate poorly with dual substituent parameters (DSP) in all possible modifications, and for meta derivatives in particular this correlation is both overpara meterized and imprecise. A much better
-
Synthesis of Sulfonyl Azides via Lewis Base Activation of Sulfonyl Fluorides and Trimethylsilyl Azide作者:John Moses、Andrew BarrowDOI:10.1055/s-0035-1561626日期:——A protocol for the efficient conversion of sulfonyl fluorides into sulfonyl azides through Lewis base activation is described. The in situ generated sulfonyl azides are efficient diazo-transfer agents, affording diazo compounds and primary azides in excellent yields.
-
Sulfur(VI) fluoride compounds and methods for the preparation thereof申请人:The Scripps Research Institute公开号:US10117840B2公开(公告)日:2018-11-06This application describes a compound represented by Formula (I): (I) wherein: Y is a biologically active organic core group comprising one or more of an aryl group, a heteroaryl aryl group, a nonaromatic hydrocarbyl group, and a nonaromatic heterocyclic group, to which Z is covalently bonded; n is 1, 2, 3, 4 or 5; m is 1 or 2; Z is O, NR, or N; X1 is a covalent bond or —CH2CH2—, X2 is O or NR; and R comprises H or a substituted or unsubstituted group selected from an aryl group, a heteroaryl aryl group, a nonaromatic hydrocarbyl group, and a nonaromatic heterocyclic group. Methods of preparing the compounds, methods of using the compounds, and pharmaceutical compositions comprising the compounds are described as well.
-
[EN] KRAS G12D INHIBITORS<br/>[FR] INHIBITEURS DE KRAS G12D申请人:MIRATI THERAPEUTICS INC公开号:WO2021041671A1公开(公告)日:2021-03-04The present invention relates to compounds that inhibit KRas G12D. In particular, the present invention relates to compounds that inhibit the activity of KRas G12D, pharmaceutical compositions comprising the compounds and methods of use therefor.本发明涉及抑制KRas G12D的化合物。具体地,本发明涉及抑制KRas G12D活性的化合物,包括这些化合物的药物组合物以及使用方法。
表征谱图
-
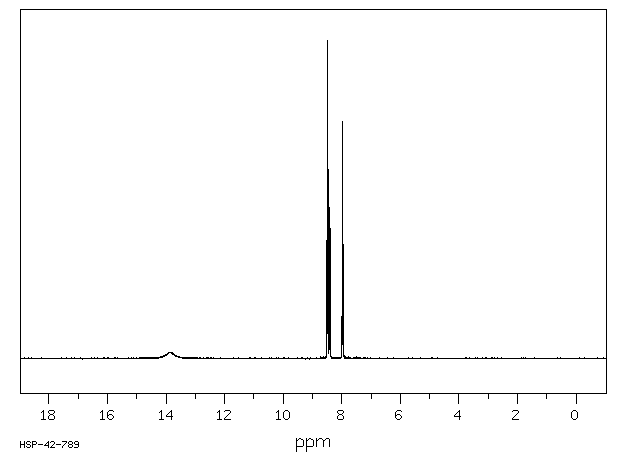
氢谱1HNMR
-
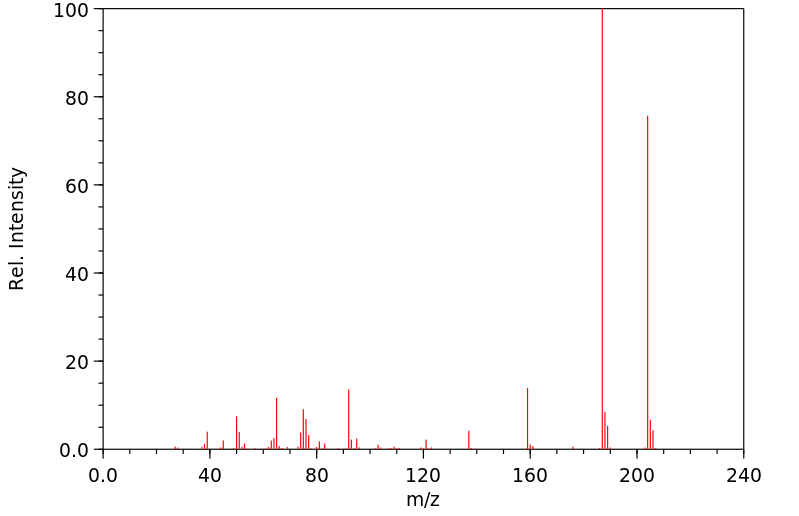
质谱MS
-
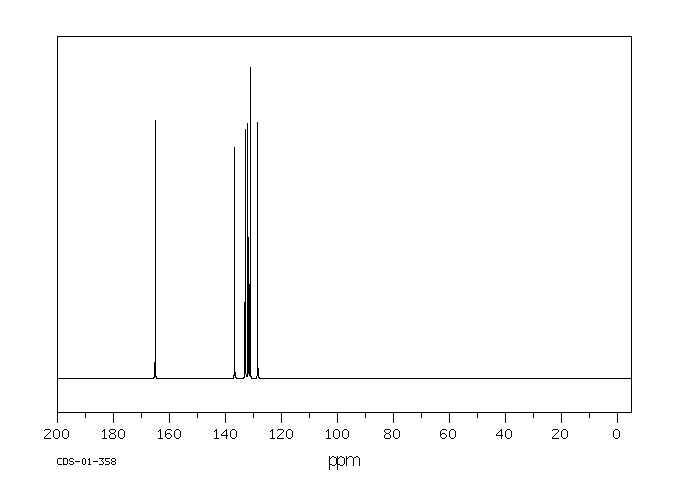
碳谱13CNMR
-
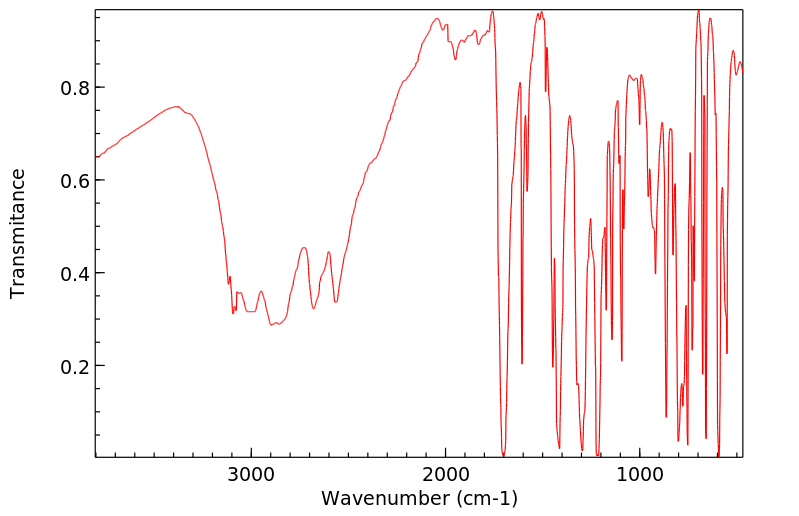
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫