3-氰基丙酸甲酯 | 4107-62-4
中文名称
3-氰基丙酸甲酯
中文别名
3-氰丙酸甲酯
英文名称
3-cyano-propionic acid methyl ester
英文别名
methyl 3-cyanopropanoate;methyl 3-cyanopropionate;ω-cyanopropionic acid methyl ester
CAS
4107-62-4
化学式
C5H7NO2
mdl
——
分子量
113.116
InChiKey
BPSKURPOKFSLHJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:216 °C
-
密度:1,08 g/cm3
-
溶解度:溶于氯仿
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:50.1
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:6.1
-
安全说明:S26,S36,S53
-
危险品运输编号:UN 3276
-
海关编码:2926909090
-
危险类别码:R20/21/22
-
包装等级:III
-
危险类别:6.1
-
危险性防范说明:P261,P280,P301+P310,P311
-
危险性描述:H301+H311+H331
-
储存条件:请将产品存放在避光、通风且干燥的地方,并密封保存。
SDS
3-氰基丙酸甲酯 修改号码:5
模块 1. 化学品
产品名称: Methyl 3-Cyanopropionate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
急性毒性(经口) 第3级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 可燃液体
吞咽会中毒。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离明火/热表面。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 食入:立即呼叫解毒中心/医生。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
3-氰基丙酸甲酯 修改号码:5
模块 2. 危险性概述
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3-氰基丙酸甲酯
百分比: >99.0%(GC)
CAS编码: 4107-62-4
俗名: 3-Cyanopropionic Acid Methyl Ester
分子式: C5H7NO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
3-氰基丙酸甲酯 修改号码:5
模块 7. 操作处置与储存
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 216 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.08
溶解度:
[水] 无资料
[其他溶剂]
溶于: 氯仿
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氰化氢(氢氰酸)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
3-氰基丙酸甲酯 修改号码:5
模块 12. 生态学信息
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第1项 毒害品。
UN编号: 3276
正式运输名称: 腈类, 液体, 有毒的, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Methyl 3-Cyanopropionate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
急性毒性(经口) 第3级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 可燃液体
吞咽会中毒。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离明火/热表面。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 食入:立即呼叫解毒中心/医生。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
3-氰基丙酸甲酯 修改号码:5
模块 2. 危险性概述
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3-氰基丙酸甲酯
百分比: >99.0%(GC)
CAS编码: 4107-62-4
俗名: 3-Cyanopropionic Acid Methyl Ester
分子式: C5H7NO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
3-氰基丙酸甲酯 修改号码:5
模块 7. 操作处置与储存
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 216 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.08
溶解度:
[水] 无资料
[其他溶剂]
溶于: 氯仿
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氰化氢(氢氰酸)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
3-氰基丙酸甲酯 修改号码:5
模块 12. 生态学信息
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第1项 毒害品。
UN编号: 3276
正式运输名称: 腈类, 液体, 有毒的, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:The Preparation of Geminally Substituted 4-Bromobutylamines. II. 4-Bromo-2,2-dialkyl- and diarylbutylamines1摘要:DOI:10.1021/ja01610a002
-
作为产物:描述:参考文献:名称:Ketene Acetals. XX. The Preparation and Properties of Cyanoketene Acetals. Some Novel Benzylation Reactions摘要:DOI:10.1021/ja01169a014
文献信息
-
Broad spectrum antibiotics
-
Structure–activity relationships for inhibitors of Pseudomonas aeruginosa exoenzyme S ADP-ribosyltransferase activity作者:Michael Saleeb、Charlotta Sundin、Öznur Aglar、Ana Filipa Pinto、Mahsa Ebrahimi、Åke Forsberg、Herwig Schüler、Mikael ElofssonDOI:10.1016/j.ejmech.2017.11.036日期:2018.1recombinant ExoS ADPRT domain. Herein, we report structure–activity relationships of this compound class by comparing a total of 51 compounds based on a thieno [2,3-d]pyrimidin-4(3H)-one and 4-oxo-3,4-dihydroquinazoline scaffolds. Improved inhibitors with in vitro IC50 values of 6 μM were identified. Importantly, we demonstrated that the most potent inhibitors block ADPRT activity of native full-length在感染期间,革兰氏阴性机会性病原体铜绿假单胞菌利用其III型分泌系统将毒素外切酶S(ExoS)转移到真核宿主细胞的细胞质中。ExoS是一种必不可少的体内毒力因子,可使铜绿假单胞菌避免吞噬作用并最终杀死宿主细胞。ExoS主要通过ADP-核糖基转移酶(ADPRT)活性引起其致病性。我们最近鉴定了具有体外IC 50的新型ExoS ADPRT抑制剂使用重组ExoS ADPRT结构域进行酶促测定时,大约20μM的核酸。在这里,我们通过比较基于噻吩并[2,3- d ]嘧啶-4(3 H)-one和4-oxo-3,4-dihydroquinazoline支架的51种化合物来报告该化合物类别的结构-活性关系。鉴定出体外IC 50值为6μM的改良抑制剂。重要的是,我们证明了最有效的抑制剂在酶促试验中能阻断铜绿假单胞菌分泌的天然全长ExoS的ADPRT活性,IC 50值为1.3μM 。该化合物类别有望成为开发新型抗菌剂的起点。
-
N-substituted prodrugs of fluorooxindoles申请人:Starrett E. John公开号:US20050203089A1公开(公告)日:2005-09-15The present invention provides novel N-substituted fluorooxindoles having the general Formula I wherein the wavy bond represents the racemate, the (R)-enantiomer or the (S)-enantiomer and m, n, p, q, A, B, D, Q, X, and Z are as defined below, or a nontoxic pharmaceutically acceptable salt or solvate thereof and are useful in the treatment of disorders which are responsive to the opening of potassium channels.
-
Lewis acid-mediated defluorinative [3+2] cycloaddition/aromatization cascade of 2,2-difluoroethanol systems with nitriles作者:Min-Tsang Hsieh、Kuo-Hsiung Lee、Sheng-Chu Kuo、Hui-Chang LinDOI:10.1002/adsc.201701581日期:2018.4.17and chemical stability, make derivatization of organic fluorine‐containing compounds by the activation of the C−F bond and subsequent functionalization quite challenging. We herein report a Lewis acid‐mediated defluorinative cycloaddition/aromatization cascade of 2,2‐difluoroethanols with nitriles as a novel synthetic method for the preparation of 2,4,5‐trisubstituted oxazoles. This reaction, which
-
[EN] CERTAIN KYNURENINE-3-MONOOXYGENASE INHIBITORS, PHARMACEUTICAL COMPOSITIONS, AND METHODS OF USE THEREOF<br/>[FR] INHIBITEURS DE KYNURÉNINE-3-MONOOXYGÉNASE, COMPOSITIONS PHARMACEUTIQUES ET LEURS PROCÉDÉS D'UTILISATION申请人:CHDI INC公开号:WO2010011302A1公开(公告)日:2010-01-28Certain chemical entities are provided herein. Pharmaceutical compositions comprising at least one chemical entity and one or more pharmaceutically acceptable vehicle. Methods of treating patients suffering from certain diseases and disorders responsive to the inhibition of KMO activity are described, which comprise administering to such patients an amount of at least one chemical entity effective to reduce signs or symptoms of the disease or disorder are disclosed. These diseases include neurodegenerative disorders such as Huntington's disease. Methods of treatment include administering at least one chemical entity as a single active agent or administering at least one chemical entity in combination with one or more other therapeutic agents. Also provided are methods for screening compounds capable of inhibiting KMO activity.
表征谱图
-
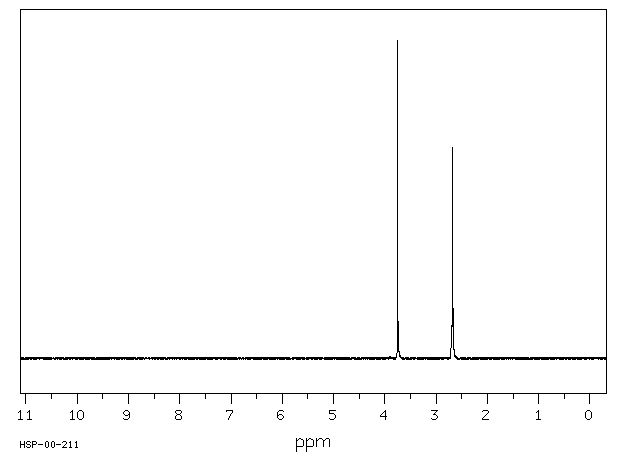
氢谱1HNMR
-
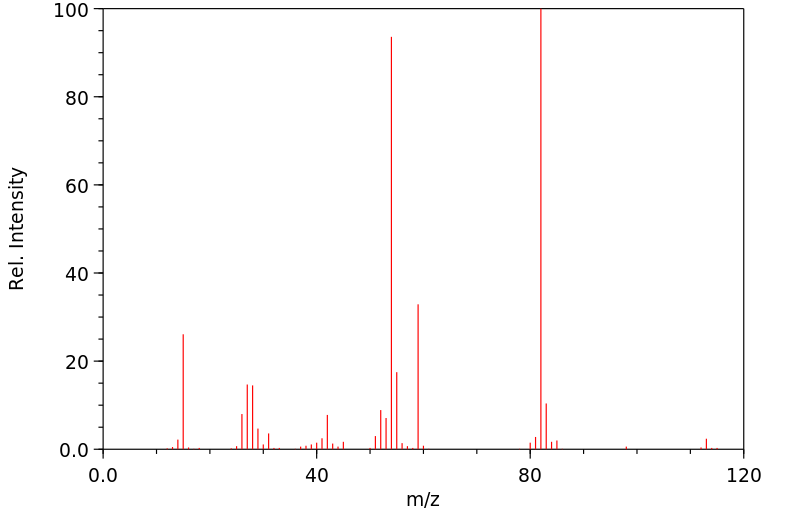
质谱MS
-
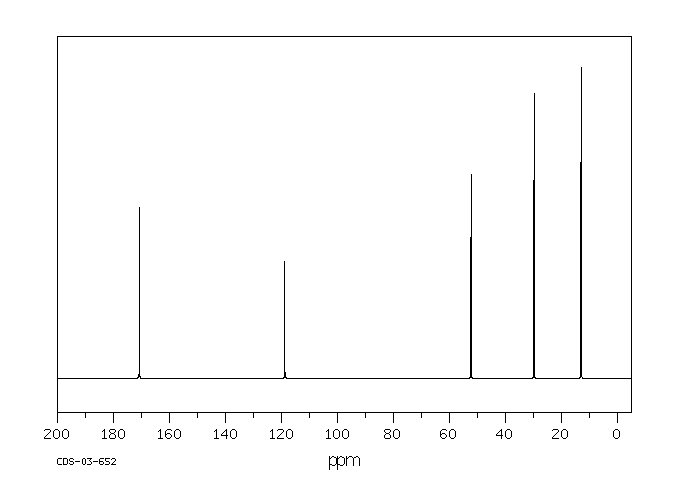
碳谱13CNMR
-
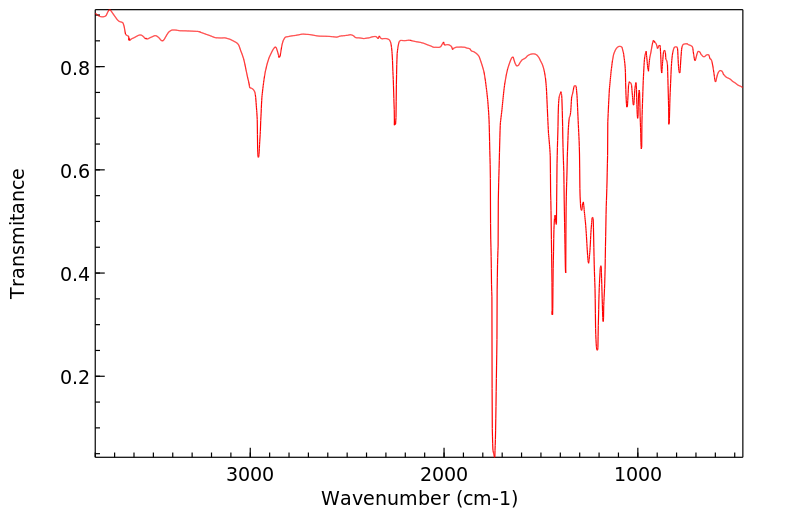
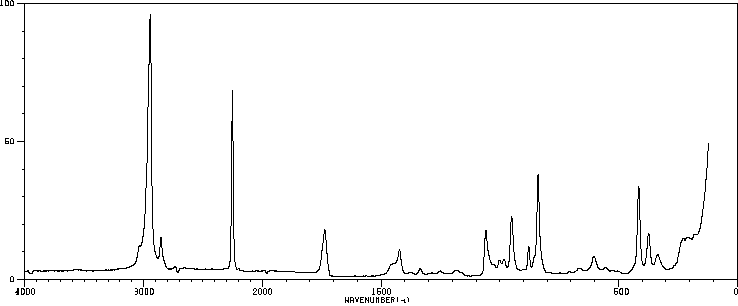
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯