3-甲氧基-9H-咔唑 | 18992-85-3
中文名称
3-甲氧基-9H-咔唑
中文别名
——
英文名称
3-methoxycarbazole
英文别名
3-methoxy-9H-carbazole;3-Methoxy-carbazol
CAS
18992-85-3
化学式
C13H11NO
mdl
MFCD00626045
分子量
197.236
InChiKey
BISIQSCKDZYPLR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:138-139 °C
-
沸点:392.2±15.0 °C(Predicted)
-
密度:1.232±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:15
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.076
-
拓扑面积:25
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:IRRITANT
-
海关编码:2933990090
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312,P302+P352,P304+P340,P330,P363,P501
-
危险性描述:H302,H312,H332
-
储存条件:室温
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-甲氧基吲哚 5-methoxylindole 1006-94-6 C9H9NO 147.177 咔唑 9H-carbazole 86-74-8 C12H9N 167.21 3-溴咔唑 3-bromo-9H-carbazole 1592-95-6 C12H8BrN 246.106 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 9H-咔唑-3-醇 3-Hydroxycarbazol 7384-07-8 C12H9NO 183.21 —— 3-bromo-6-methoxy-9H-carbazole 200289-73-2 C13H10BrNO 276.132 —— 3-methoxy-9-methyl-9H-carbazole 152404-99-4 C14H13NO 211.263 —— 3-hydroxy-9-methylcarbazole 52602-27-4 C13H11NO 197.236 —— 1-(3-methoxy-9H-carbazol-9-yl) ethan-1-one 173312-32-8 C15H13NO2 239.274 —— 3-methoxy-9-phenylcarbazole 1417618-07-5 C19H15NO 273.334 —— 6-methoxy-9-methyl-9H-carbazole-3-boronic acid 1427160-85-7 C14H14BNO3 255.081 —— 9-phenyl-9H-carbazol-3-ol 115552-06-2 C18H13NO 259.307
反应信息
-
作为反应物:描述:参考文献:名称:Structure-activity relationship of bromoeudistomin D, a powerful Ca2+ releaser in skeletal muscle sarcoplasmic reticulum摘要:Bromoeudistomin D and 9-methyl-7-bromoeudistomin D which have a beta-carboline skeleton are powerful Ca2+ releasers from skeletal muscle sarcoplasmic reticulum exhibiting caffeine-like properties. We examined the effects of bromoeudistomin D analogues on Ca(2+)-induced Ca2+ release from skeletal muscle sarcoplasmic reticulum. Among bromoeudistomin D analogues, the Ca(2+)-releasing activities of carboline derivatives were higher than those of carbazole derivatives, suggesting that a carboline skeleton is significantly important for the manifestation of Ca(2+)-releasing activity and Ca2+ sensitivity of Ca(2+)-induced Ca2+ release. On the contrary, the analogues which have a carbazole skeleton and bromine at C-6 inhibit both Ca(2+)- and caffeine-induced Ca2+ release. 9-Methyl-substitution of the analogue elevated its Ca(2+)-releasing activity. Moreover, there is a close correlation between the enhancement of [3H]ryanodine binding to sarcoplasmic reticulum by the analogues and the activation of Ca2+ release by them. Bromoudistomin D analogues may provide valuable information about the structure-function relationship of the ryanodine receptor/Ca2+ release channels in skeletal muscle sarcoplasmic reticulum.DOI:10.1016/0922-4106(95)90040-3
-
作为产物:参考文献:名称:三唑嗪-重氮甲基嗪价异构化。[1,2,3]三唑并[1,5- a ]吡啶和2-重氮甲基吡啶摘要:[1-2,3]三唑并[1,5- a ]吡啶1T和6T的价异构体2-重氮甲基吡啶1D和6D已通过温和的快速真空热解法在约2080 cm –1处直接观察到( FVP)在200–600°C的温度下进行红外光谱分析。经计算确认约。由[1,2,3]三唑并[1,5- a ]吡啶1T形成2-重氮甲基吡啶1D的17 kcal / mol势垒,重氮化合物约 在三唑之上5 kcal / mol。在较高温度范围(400–600°C)中,2-重氮甲基吡啶1D消除N 2并形成2-吡啶基卡宾2和重排为1-氰基环戊二烯4。2-重氮甲基吡啶1D在20–90°C下与四氰基乙烯(TCNE)进行1,3-偶极环加成反应,生成3-(2-吡啶基)环丙烷四甲腈11和3-(三氰基乙烯基)-[1,2,3]三唑[1] ,5- a ]吡啶13T通过未观察到的吡唑啉10和12。三唑13T的FVP在2080 cm –1处具有对应的重氮化合物13D的IR吸收。DOI:10.1021/acs.joc.5b02639
文献信息
-
The reactions of some tetrahydro-β-carbolines, of hexahydroazepino[3,4-b]indoles, and of tetrahydrocarbazolones with arenesulphonyl azides作者:A. Sydney Bailey、Marazban H. VandrevalaDOI:10.1039/p19800001512日期:——9-dimethyl-1,2,3,4-tetrahydro-β-carboline react with arenesulphonyl azides forming indoline-3-spiropyrrolidines; 2,10-dimethyl-3,4,5,10-tetrahydroazepino[3,4-b]indol-1 (2H)-one and 2,10-dimethyl-1,2,3,4,5,10-hexahydroazepino[3,4-b]indole react to form indoline-3-spiropiperidines. 9-Methyl-2-oxo-tetrahydrocarbazole reacts with p-chlorobenzenesulphonyl azide to form 1-methyl-2-p-chlorophenylsulphonylimino
-
Enantioconvergent Alkylations of Amines by Alkyl Electrophiles: Copper-Catalyzed Nucleophilic Substitutions of Racemic α-Halolactams by Indoles作者:Agnieszka Bartoszewicz、Carson D. Matier、Gregory C. FuDOI:10.1021/jacs.9b07875日期:2019.9.18classic SN2 reaction of an amine with an alkyl electrophile, both with respect to reactivity and to enantioselectivity. In this study, we describe the development of a user-friendly method (reaction at room temperature, with commercially available catalyst components) for the enantioconvergent nucleophilic substitution of racemic secondary alkyl halides (α-iodolactams) by indoles. Mechanistic studies are
-
Copper(II) catalyzed aromatization of tetrahydrocarbazole: An unprecedented protocol and its utility towards the synthesis of carbazole alkaloids作者:Bhakti A. Dalvi、Pradeep D. LokhandeDOI:10.1016/j.tetlet.2018.01.061日期:2018.5protocol for the aromatization of tetrahydrocarbazole is described by using catalytic copper(II) chloride dihydrate in DMSO. This newly established methodology has utilized towards the synthesis of naturally occurring carbazole alkaloids, namely 3-methylcarbazole, 3-formyl carbazole, glycozoline, glycozolicine and clauszoline-K. In addition, the protocol is generalized for the aromatization of N-substituted
-
Transition‐Metal‐Catalyzed Alkylations of Amines with Alkyl Halides: Photoinduced, Copper‐Catalyzed Couplings of Carbazoles作者:Alex C. Bissember、Rylan J. Lundgren、Sidney E. Creutz、Jonas C. Peters、Gregory C. FuDOI:10.1002/anie.201301202日期:2013.5.3N‐alkylations of carbazoles with a variety of secondary and hindered primary alkyl iodides can be achieved by using a simple precatalyst (CuI) under mild conditions (0 °C) in the presence of a Brønsted base; at higher temperature (30 °C), secondary alkyl bromides also serve as suitable coupling partners. A Li[Cu(carbazolide)2] complex has been crystallographically characterized, and it may serve as
-
Heterogeneous photocatalysis of azides: extending nitrene photochemistry to longer wavelengths作者:Ignacio D. Lemir、Juan E. Argüello、Anabel E. Lanterna、Juan C. ScaianoDOI:10.1039/d0cc04118a日期:——The photodecomposition of azides to generate nitrenes usually requires wavelengths in the <300 nm region. In this study, we show that this reaction can be readily performed in the UVA region (368 nm) when catalyzed by Pd-decorated TiO2. In aqueous medium the reaction leads to amines, with water acting as the H source; however, in non-protic and non-nucleophilic media, such as acetonitrile, nitrenes
表征谱图
-
氢谱1HNMR
-
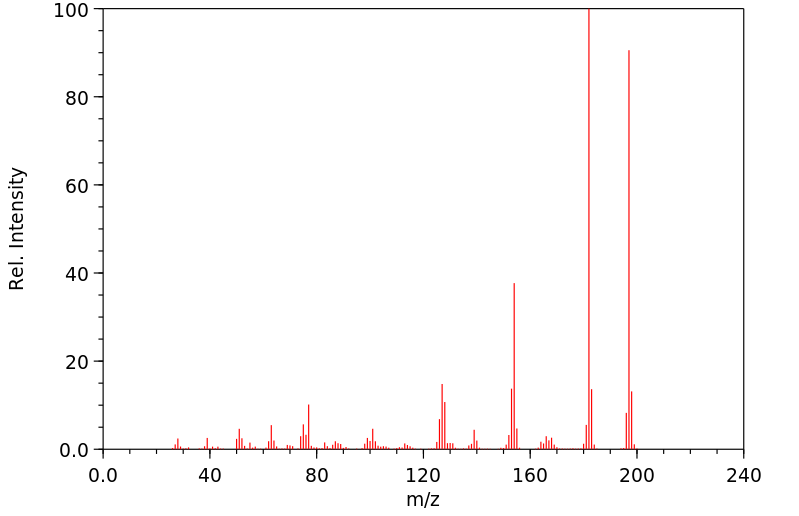
质谱MS
-
碳谱13CNMR
-
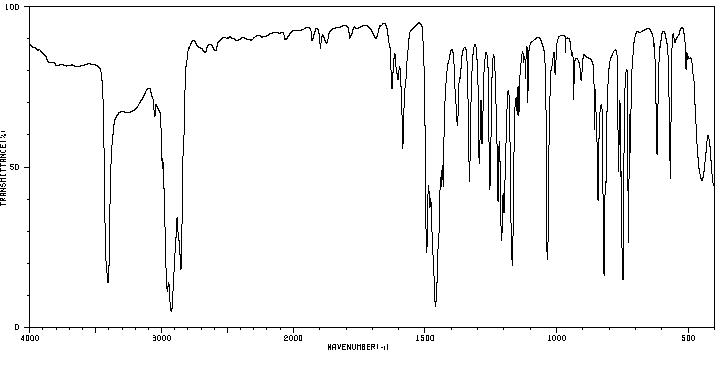
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3