硫代异氰酸苯酯 | 103-72-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:−21 °C(lit.)
-
沸点:218 °C(lit.)
-
密度:1.132 g/mL at 20 °C(lit.)
-
闪点:190 °F
-
溶解度:水:不溶
-
介电常数:10.7(20℃)
-
LogP:3.280
-
保留指数:1163
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:44.4
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T,N
-
安全说明:S16,S23,S26,S29,S33,S36,S36/37/39,S45,S60,S61,S62,S9
-
危险类别码:R23/24/25,R34,R42/43,R63
-
WGK Germany:3
-
海关编码:2930909090
-
危险品运输编号:UN 1993 3/PG 2
-
危险类别:6.1
-
RTECS号:NX9275000
-
包装等级:II
-
危险标志:GHS05,GHS06,GHS08
-
危险性描述:H301,H314,H317,H334
-
危险性防范说明:P261,P280,P301 + P310,P305 + P351 + P338,P310
-
储存条件:储存注意事项:应储存在阴凉、干燥且通风良好的库房中,并远离火种与热源。保持容器密封。避免与氧化剂、酸类、碱类及食用化学品混存,以防混合存储引发危险。需配备相应种类和数量的消防设备。储存区还应配置泄漏应急处理设施以及适当的回收材料。
制备方法与用途
化学性质
无色或黄色液体,具有强烈刺激性气味。不溶于水,但能溶解在乙醇和乙醚中。
用途
常作为有机合成原料,并用于多种应用:
- 将二苯硫脲加入盐酸中加热溶解。
- 蒸馏出生成的异硫氰酸苯酯。
- 在油状产物中加入等体积水进行洗涤,分离水层。
- 使用稀碱液进一步洗涤。
- 经多次大量水洗后,用无水氯化钙干燥。
- 收集120-121℃(4.66kPa)的馏分即为成品。
类别
有毒物品
毒性分级
高毒
急性毒性
腹腔注射-大鼠 LD50: 150 毫克/公斤;口服-小鼠 LD50: 87 毫克/公斤。
可燃性危险特性
遇明火可燃,高温分解时会释放氰化物及氧化硫气体。
储运特性
需存放在通风、低温和干燥的库房中,并与氧化剂、酸类以及食品添加剂分开存放。
灭火方法
使用干粉、泡沫或二氧化碳灭火。避免使用酸碱灭火剂。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-溴苯基异硫氰酸酯 4-Bromophenyl isothiocyanate 1985-12-2 C7H4BrNS 214.085 对甲苯异硫氰酸酯 1-isothiocyanato-4-methylbenzene 622-59-3 C8H7NS 149.216 异氰酸苯酯 phenyl isocyanate 103-71-9 C7H5NO 119.123 4-甲氧基苯基 异硫氰酸酯 4-Methoxyphenyl isothiocyanate 2284-20-0 C8H7NOS 165.216 2-氯-5-异氰酸硝基苯 1,3-Diphenylcarbodiimide 622-16-2 C13H10N2 194.236 二氯代苯胩 phenyl isocyanodichloride 622-44-6 C7H5Cl2N 174.029 —— 1-bromo-2-isothiocyanatobenzene 64217-61-4 C7H4BrNS 214.085 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 对甲苯异硫氰酸酯 1-isothiocyanato-4-methylbenzene 622-59-3 C8H7NS 149.216 异氰酸苯酯 phenyl isocyanate 103-71-9 C7H5NO 119.123 3-氯异硫氰酸苯酯 3-chlorophenyl-isothiocyanate 2392-68-9 C7H4ClNS 169.634 4-二甲氨基苯基硫代异氰酸酯 4-isothiocyanato-N,N-dimethylaniline 2131-64-8 C9H10N2S 178.258 —— N-((methylimino)methylene)aniline 4172-91-2 C8H8N2 132.165 —— phenyl-carboximidoyl fluoride 1544-84-9 C7H5F2N 141.12 2-氯-5-异氰酸硝基苯 1,3-Diphenylcarbodiimide 622-16-2 C13H10N2 194.236 二氯代苯胩 phenyl isocyanodichloride 622-44-6 C7H5Cl2N 174.029 2-氯苯基异氰酸酯 2-chlorophenylisothiocyanate 2740-81-0 C7H4ClNS 169.634 —— 1-bromo-2-isothiocyanatobenzene 64217-61-4 C7H4BrNS 214.085
反应信息
-
作为反应物:参考文献:名称:一种将异氰酸酯还原为异氰酸酯的新方法摘要:在温和的条件下,二苯基叔丁基甲硅烷基锂和三氯硅烷三乙胺都能将异氰酸酯高产率地还原为异氰酸酯。DOI:10.1039/c39820000942
-
作为产物:参考文献:名称:Self-Resolution of Alcohol Problems in Young Adulthood: A Process of Securing Solid Ground摘要:定量研究结果表明,年轻成年人在解决酒精问题时并没有参加支持团体或正式治疗计划。然而,研究人员未能充分解释这个年龄群体中的自我解决过程。因此,作者们使用了扎根理论来更好地阐明年轻成年人为什么以及如何自我解决酒精问题。研究结果表明,在年轻成年人中自我解决酒精问题涉及一个寻找和确保坚实基础的时间过程。这个过程是由个体经历摇摇欲坠的情况引发的,最终开始失去平衡。这些累积事件导致年轻成年人追求个人愿景,并在坚实基础上找到安全立足点,尽管在前进的过程中可能会遇到一些崎岖的地形。DOI:10.1177/104973202129120115
-
作为试剂:描述:2,4,6-三溴甲基三甲基苯 在 硫代异氰酸苯酯 、 三乙胺 作用下, 以 二氯甲烷 、 苯 为溶剂, 反应 39.0h, 生成 [3,5-Bis(diphenylphosphinothioylmethyl)-2,4,6-trimethylphenyl]methyl-diphenyl-sulfanylidene-lambda5-phosphane参考文献:名称:氨基膦的烷基化:亚氨基膦的新合成摘要:亚氨基膦是通过易得的 N,P,P-三取代氨基膦与反应性烷基卤化物的 P-烷基化和进一步去质子化制备的。DOI:10.1055/s-2003-38730
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] CRBN LIGANDS AND USES THEREOF<br/>[FR] LIGANDS CRBN ET LEURS UTILISATIONS申请人:KYMERA THERAPEUTICS INC公开号:WO2019140387A1公开(公告)日:2019-07-18The present invention provides compounds, compositions thereof, and methods of using the same for the inhibition of CRBN, and the treatment of CRBN-mediated disorders.本发明提供了化合物、其组合物以及使用这些化合物抑制CRBN并治疗CRBN介导的疾病的方法。
-
A Novel Route to Isoquinoline[2,1-g][1,6]naphthyridine, Pyrazolo[5,1-a] isoquinoline and Pyridazino[4´,5´:3,4]pyrazolo[5,1-a]isoquinoline Derivatives With Evaluation of Antitumor Activities作者:Hamdi M. Hassaneen、Wagnat W. Wardkhan、Yasmin Sh. MohammedDOI:10.5560/znb.2013-3101日期:2013.8.1
(E)-2-Chloro-3-(2-cyanovinyl)-9,10-dimethoxy-4-oxo-6,7-dihydro-4H-pyrido[2,1-a] isoquinoline- 1-carbonitrile (5) was obtained by treatment of the 2-chloro-3-formylpyrido[2,1-a]isoquinoline derivative 3 with 2-(triphenylphosphoranylidene)acetonitrile (4). Treatment of 5 with sodium azide afforded the corresponding azido compound 6 which could be reduced by sodium dithionite to compound 7. A novel isoquinolino[2,1-g][1,6]naphthyridine derivative 11 was obtained by the reaction of phenyl isothiocyanate with the phosphorane compound 8, which was prepared by the reaction of compound 6 with triphenylphosphine. Treatment of 5 with amines 12a-c and thiophenols 14a-c in refluxing ethanol afforded the corresponding substitution products 13a-c and 15a-c, respectively. Also, the reaction of 1 with a-oxo hydroxamoyl chlorides 16 was reinvestigated, and the synthesized pyrazoloisoquinolines 19a-f and pyridazinopyrazoloisoquinolines 20a, e were screened for their in vitro antitumor activities.
(E)-2-氯-3-(2-氰基乙烯基)-9,10-二甲氧基-4-氧代-6,7-二氢-4H-吡啶并[2,1-a]异喹啉-1-甲腈 (5) 由 2-(三苯基膦亚基)乙腈 (4) 处理 2-氯-3-甲酰基吡啶并[2,1-a]异喹啉衍生物 3 而得。用叠氮化钠处理 5 后可得到相应的叠氮化合物 6,该化合物可通过亚硫酸钠还原成化合物 7。异硫氰酸苯酯与磷烷化合物 8 反应得到了新型异喹啉并[2,1-g][1,6]萘啶衍生物 11,后者是由化合物 6 与三苯基膦反应制备的。在回流乙醇中将 5 与胺 12a-c 和噻吩酚 14a-c 处理,可分别得到相应的取代产物 13a-c 和 15a-c。此外,还重新研究了 1 与 a-氧代羟基氨基甲酰氯 16 的反应,并对合成的吡唑并异喹醇类 19a-f 和哒嗪并异喹醇类 20a, e 进行了体外抗肿瘤活性筛选。 -
Reactivity of Thiosemicarbazides with Redox Active Metal Ions: Controlled Formation of Coordination Complexes versus Heterocyclic Compounds作者:Elena López-Torres、Jonathan R. DilworthDOI:10.1002/chem.200801446日期:2009.3.9Careful metal selection: Control of the reaction conditions of CuII and SnIV with Me2NNHCSNHPh permits the selective synthesis of metal complexes or a range of heterocyclic compounds generated by the oxidative coupling of two thiosemicarbazides (see figure).
-
Studying Products of Hydrazine Interaction with Isothiocyanates by Means of Chromatography and Mass Spectrometry作者:A. V. Ul’yanov、K. E. Polunin、I. A. Polunina、A. K. BuryakDOI:10.1134/s0036024421050290日期:2021.5compounds in real-time and delayed modes are optimized. The physicochemical characteristics of the sorption of thiosemicarbazides are determined. The decomposition and fragmentation of their metastable protonated molecules are studied. Schemes are proposed for the formation of fragmented and characteristic thiosemicarbazide ions in different modes of ionization.
表征谱图
-
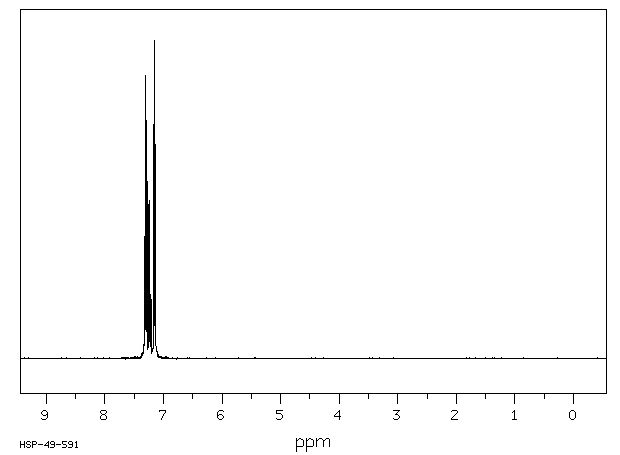
氢谱1HNMR
-
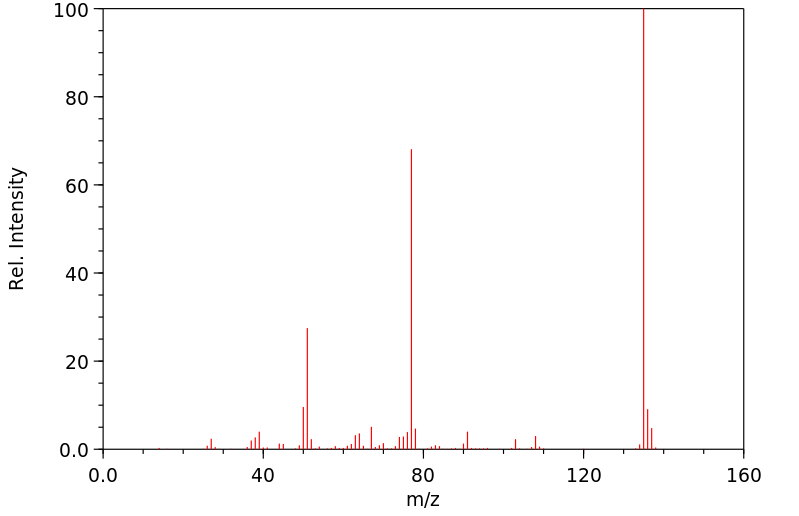
质谱MS
-
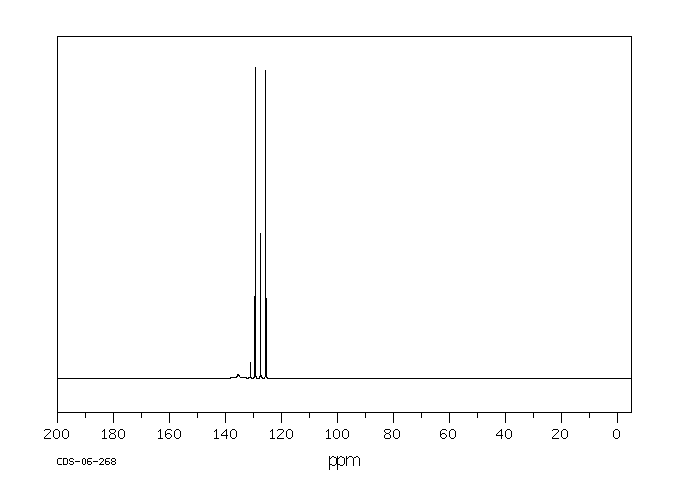
碳谱13CNMR
-
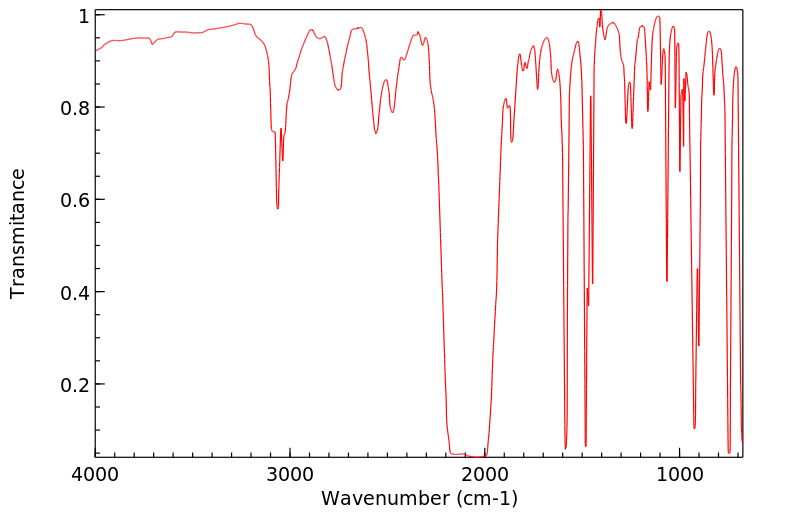
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息