1,2,3,4-tetraphenyl-1,4-butane dione | 21072-57-1
分子结构分类
中文名称
——
中文别名
——
英文名称
1,2,3,4-tetraphenyl-1,4-butane dione
英文别名
meso-Didesil;(2S,3R)-1,2,3,4-tetraphenylbutane-1,4-dione
CAS
21072-57-1
化学式
C28H22O2
mdl
——
分子量
390.481
InChiKey
SLHKSCFJQMEDTP-WMPKNSHKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:572.8±50.0 °C(Predicted)
-
密度:1.161±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):6.2
-
重原子数:30
-
可旋转键数:7
-
环数:4.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为产物:描述:(3S,6R)-3,4,5,6-Tetraphenyl-3,6-dihydro-[1,2]dioxine 在 氢氧化钾 作用下, 以 甲醇 为溶剂, 生成 1,2,3,4-tetraphenyl-1,4-butane dione参考文献:名称:Rio,G.; Berthelot,J., Bulletin de la Societe Chimique de France, 1971, p. 2938 - 2942摘要:DOI:
文献信息
-
Rhodium-catalyzed synthesis of 2,3-diaryl-1,4-diketones via oxidative coupling of benzyl ketones using α-thioketone oxidizing reagent作者:Mieko Arisawa、Guangzhe Li、Masahiko YamaguchiDOI:10.1016/j.tetlet.2012.12.107日期:2013.3no)benzene (dppBz) catalyzed the oxidative coupling reaction of aryl benzyl ketones giving 2,3-diaryl-1,4-diketones in high yields. 3,3-Dimethyl-1-methylthio-2-butanone was used as the oxidizing reagent, which was converted to 3,3-dimethyl-2-butanone and dimethyl disulfide. Rhodium enolates were catalytically formed from ketones, which underwent oxidative coupling using an organosulfur reagent.
-
Modulation of Lifetimes and Diastereomeric Discrimination in Triplet-Excited Substituted Butane-1,4-diones through Intramolecular Charge-Transfer Quenching作者:J. N. Moorthy、S. L. Monahan、R. B. Sunoj、J. Chandrasekhar、C. BohneDOI:10.1021/ja9818708日期:1999.4.1lifetimes have been determined for the diastereomers of a broad set of butane-1,4-dione derivatives (1−3). A remarkable dependence of lifetimes on conformational preferences is revealed in that the lifetimes are shorter for the meso diastereomers of 1−3 than those for the racemic ones. The intramolecular β-phenyl quenching is promoted in the case of meso diastereomers by virtue of the gauche relationship
-
CHIKASHITA HIDENORI; MIYAZAKI MAKOTO; ITOH KAZUYOSHI, J. CHEM. SOC. PERKIN TRANS.,(1987) N 4, 699-706作者:CHIKASHITA HIDENORI、 MIYAZAKI MAKOTO、 ITOH KAZUYOSHIDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
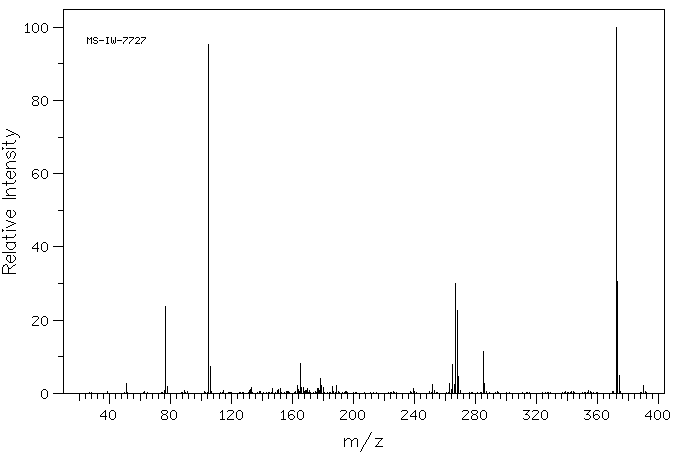
质谱MS
-
碳谱13CNMR
-
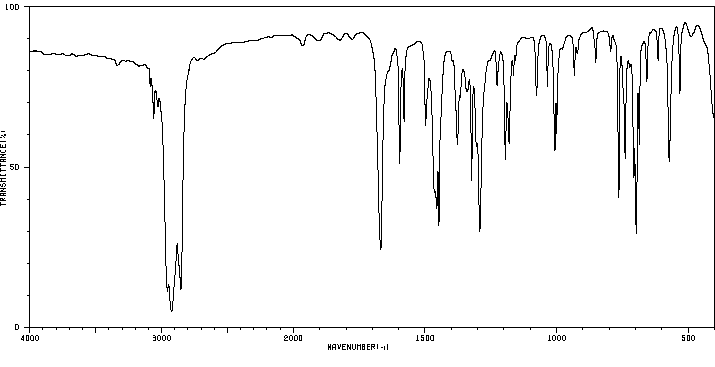
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
苯基(2,4,5-三苯基-2-环戊烯-1-基)甲酮
肠二醇
珠子草素
开环异落叶松树脂酚
安五脂素
外消旋肠二醇-13C3
去甲二氢愈创木酸
半去甲二氢愈创木酸
二甲基2,5-二苯基-3,4-二氢-2H-吡咯-3,4-二羧酸酯
二氢荜澄茄脂素
9,9'-二-O-(E)-阿魏酰开环异落叶松脂素
5,6-二甲基-5-苯基-1,3-环己二烯
4-[4-(4-羟基-3-甲氧基苯基)-2,3-二甲基丁基]-2-甲氧基苯酚
2-甲氧基-1,2,3,4-四苯基丁烷-1,4-二酮
2,6-二甲氧基-4-羟基苯甲醛
2,6-二甲氧基-4-[(2R,3R)-4-甲氧基-3-[(7-甲氧基-1,3-苯并二噁唑-5-基)甲基]-2-(甲氧基甲基)丁基]苯酚
2,3-双[(4-羟基-3-甲氧基苯基)甲基]丁烷-1,4-二醇
2,3-双[(3,4-二甲氧基苯基)甲基]丁烷-1,4-二醇
2,3-双[(3,4-二甲氧基苯基)甲基]丁二酸
2,3-双(4-羟基-3-甲氧基苄基)琥珀酸二甲酯
2,3-双(3,4-二甲氧基苄基)-4-甲氧基-4-氧代丁酸
2,3-二苄基丁烷-1,4-二醇
2,3-二甲基-1,4-二苯基丁烷-2,3-二醇
2,3-二甲基-1,4-二苯基丁烷-1,4-二酮
2,3-二异丙基-1,2-二苯基-1,4-丁二酮
2,3-二[(3-羟基苯基)甲基]丁烷-1,4-二醇
2,2,3,3-四甲基-1,4-二苯基丁烷-1,4-二酮
1,4-丁烷二酮
1,2,3,4-四苯基环戊烷
1,2,3,4-四苯基丁烷
1,2,3,4-四苯基-1,4-丁烷二酮
1,2,3,4-四-(4-甲氧基-苯基)-丁烷-1,4-二酮
1,1'-[(2S,3S)-2,3-双(甲氧基甲基)-1,4-丁二基]双[3,4-二甲氧基苯]
1,1'-(2,3-二甲基-1,4-丁烷二基)二(3,4-二甲氧基苯)
1,1',2,2',3,3'-六苯基-1,1'-联(2-环丙烯)
(3,3,4,4-四甲基-2-苯基环丁烯-1-基)苯
(2R,3S)-1,4-二(4-羟基-3-甲氧基苯基)-2,3-二甲基丁烷-1-酮
(-)-二氢愈创木脂酸
(+)-开环异落叶松树脂酚
(2S,3R)-2,3-Bis-(3,5-di-tert-butyl-4-hydroxy-benzyl)-butane-1,4-diol
(3-{1-[(E)-2-(4-Methoxy-phenyl)-1-methyl-vinyl]-3,3-dimethyl-1,3-dihydro-isobenzofuran-1-yl}-propyl)-dimethyl-amine
(6-Benzoyl-1,4-diphenyl-3,6-di-p-tolyl-tetrahydro-isoxazolo[4,3-c]isoxazol-3-yl)-phenyl-methanone
2-(1,3-Diphenylpropan-2-yl)-2-methylpropane-1,3-diol
1-hydroxy-6-(3-oxo-3-phenylpropyl)-4,4-diphenyl-2,3-dioxabicyclo[4.3.0]nonan-7-one
2-[2-(3,4-dimethoxy-phenyl)-1-methyl-ethyl]-3-phenyl-oxaziridine
1,3,5-triphenyl-pyrrolidine-2,2,4-tricarboxylic acid trimethyl ester
3-Methyl-1-phenyl-cyclopropane-1,2-dicarboxylic acid diethyl ester
2-Benzoyl-3-(4-methoxy-phenyl)-5-oxo-5-p-tolyl-pentanoic acid ethyl ester