4-氨基喹唑啉 | 15018-66-3
中文名称
4-氨基喹唑啉
中文别名
喹啉-4-胺;4-喹唑啉胺
英文名称
4-Aminoquinazoline
英文别名
quinazolin-4-amine;quinazolin-4-ylamine
CAS
15018-66-3
化学式
C8H7N3
mdl
MFCD00092016
分子量
145.164
InChiKey
DRYRBWIFRVMRPV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:274-275℃
-
沸点:328.3±17.0 °C(Predicted)
-
密度:1.292±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:51.8
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
海关编码:2933990090
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H317,H319
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Quinazolin-4-ylamine
Synonyms: 4-Aminoquinazoline
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Quinazolin-4-ylamine
CAS number: 15018-66-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H7N3
Molecular weight: 145.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Quinazolin-4-ylamine
Synonyms: 4-Aminoquinazoline
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Quinazolin-4-ylamine
CAS number: 15018-66-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H7N3
Molecular weight: 145.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 喹唑啉 quinazoline 253-82-7 C8H6N2 130.149 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-肼基喹唑啉 quinazolin-4-ylhydrazine 36075-44-2 C8H8N4 160.178 —— N1,N1-dimethyl-N2-(quinazolin-4-yl)formamidine 68906-17-2 C11H12N4 200.243 —— N-quinazolin-4-yl-acetamide 82435-99-2 C10H9N3O 187.201 —— 4-benzylaminoquinazoline 100818-54-0 C15H13N3 235.288 —— N-(4-methoxyphenyl)quinazolin-4-amine 34932-32-6 C15H13N3O 251.288 —— N1,N1-dimethyl-N2-(quinazolin-4-yl)acetamidine 457072-59-2 C12H14N4 214.27 —— 1-phenyl-3-(quinazolin-4-yl) urea —— C15H12N4O 264.286 —— N1,N1-dimethyl-N2-(quinazolin-4-yl)propionamidine 457072-60-5 C13H16N4 228.297
反应信息
-
作为反应物:参考文献:名称:(E)-4-(2-(吡啶-2-基亚甲基)肼基)喹唑啉与非甾体抗炎药双氯芬酸钠的Zn(II)配合物:结构,DNA结合和光裂解研究,抗氧化活性和与白蛋白的相互作用。摘要:在不存在或存在非甾体类抗炎药双氯芬酸钠的情况下,进行了新型喹唑啉(E)-4-(2-(吡啶-2-基亚甲基)肼基)喹唑啉(L)与Zn 2+的相互作用。(Nadicl)并形成络合物[Zn(L)2 ](NO 3)2 ·MeOH(1 ·MeOH)和[Zn(L)(dicl-O)2 ]·MeOH(2 ·MeOH),分别。两种配合物通过IR和1 H NMR光谱以及单晶X射线晶体学表征。在这些复合物中,L通过喹唑啉,和吡啶氮原子三齿配位于Zn(II)。已经进行了有关化合物对小胸腺(CT)DNA和超螺旋环状pBluescript KS II质粒DNA(pDNA)行为的进一步研究。配合物可以通过嵌入与CT DNA结合,配合物1的结合亲和力比2高。复合物可在不存在或存在UVA,UVB或可见光照射的情况下切割pDNA,最活跃的pDNA裂解剂是化合物1。计算化合物对牛血清白蛋白的结合常数,并确定化合物较喜欢结合的白蛋DOI:10.1016/j.jinorgbio.2020.111194
-
作为产物:参考文献:名称:肌酐新异构体的合成及其在食品诱变剂2-氨基-1-甲基-6-苯基-1 H-咪唑并[4,5- b ]吡啶(PHIP)中的应用摘要:的碱诱导的环化Ñ '-cyanomethyl- ñ '甲基脲给出1-甲基-4-氨基-咪唑-2-酮,这反过来又被冷凝与3-羟基-2- phenylacrolein,得到咪唑并[4,5 - b ]吡啶,其被转换成2-氨基-1-甲基-6-苯基-1 ħ -咪唑并[4,5- b ]吡啶(PHIP)。DOI:10.1016/j.tetlet.2009.07.091
文献信息
-
[EN] AMINOQUINAZOLINE DERIVATIVES AND THEIR SALTS AND METHODS OF USE<br/>[FR] DÉRIVÉS D'AMINOQUINAZOLINE ET LEURS SELS ET PROCÉDÉS D'UTILISATION申请人:SUNSHINE LAKE PHARMA CO LTD公开号:WO2013071697A1公开(公告)日:2013-05-23The present invention relates to the field of medicine. Provided herein are aminoquinazoline derivatives, their salts and pharmaceutical formulations useful in modulating the protein tyrosine kinase activity, and in modulating inter-and/or intra-cellular signaling. Also provided herein are pharmaceutically acceptable compositions comprising the aminoquinazoline compounds and methods of using the compositions in the treatment of hyperproliferative disorders in mammals, especially humans.
-
AMINOQUINAZOLINE DERIVATIVES AND THEIR SALTS AND METHODS OF USE申请人:SUNSHINE LAKE PHARMA CO., LTD.公开号:US20140228361A1公开(公告)日:2014-08-14The present invention relates to the field of medicine. Provided herein are aminoquinazoline derivatives, their salts and pharmaceutical formulations useful in modulating the protein tyrosine kinase activity, and in modulating inter- and/or intra-cellular signaling. Also provided herein are pharmaceutically acceptable compositions comprising the aminoquinazoline compounds and methods of using the compositions in the treatment of hyperproliferative disorders in mammals, especially humans.
-
[EN] QUINAZOLINE DERIVATIVES<br/>[FR] DERIVES DE QUINAZOLINE申请人:ASTRAZENECA AB公开号:WO2005028469A1公开(公告)日:2005-03-31The invention concerns quinazoline derivatives of Formula (I): wherein each of R1, X1, R2, R3, R5, n and m have any of the meanings defined in the description; processes for their preparation, pharmaceutical compositions containing them and their use in the manufacture of a medicament for use as an antiproliferative agent in the prevention or treatment of tumours which are sensitive to inhibition of EGF and erbB receptor tyrosine kinases.
-
[EN] MONO AND BICYCLIC RING BORONIC ACID, ESTER AND SALT COMPOUNDS AS INHIBITORS OF P97 COMPLEX<br/>[FR] COMPOSÉS D'ESTER, DE SEL ET D'ACIDE BORONIQUE À NOYAU MONOCYCLIQUE ET BICYCLIQUE EN TANT QU'INHIBITEURS DE COMPLEXE P97申请人:CLEAVE BIOSCIENCES INC公开号:WO2016200840A1公开(公告)日:2016-12-15Monocyclic or bicyclic ring boronic acid or ester compounds having an arylalkyi amine substituent at the 4 position and a substituted 5:6 bicyclic group at the 2 position of the mono or bicyclic ring boronic acid or ester as well as optional aliphatic, functional and/or aromatic components substituted at other positions of the mono or bicyclic boronic acid or ester compounds are disclosed. These compounds are inhibitors of the AAA proteasome complex containing p97 and are effective medicinal agents for treatment of diseases associated with the Valosin containing protein and p97 bioactivity such as cancer. Formula (I).
-
[EN] CONDENSED PYRIDINES AND PYRIMIDINES AND THEIR USE AS ALK-5 RECEPTOR LIGANDS<br/>[FR] PYRIDINES ET PYRIMIDINES CONDENSEES ET LEUR UTILISATION EN TANT QUE LIGANDS DU RECEPTEUR ALK-5申请人:SMITHKLINE BEECHAM CORP公开号:WO2004065392A1公开(公告)日:2004-08-05Therapeutically active substituted quinoline and quinazoline compounds derivatives of formula (I) wherein X is N or CH, Y is NH, N (alkyl) or NH-CH2, and R2 and R3 are specified heterorings, the use thereof in therapy, particularly in the treatment or prophylaxis of disorders characterised by overexpression of transforming growth factor β (TGF-β), and pharmaceutical compositions for use in such therapy are disclosed.
表征谱图
-
氢谱1HNMR
-
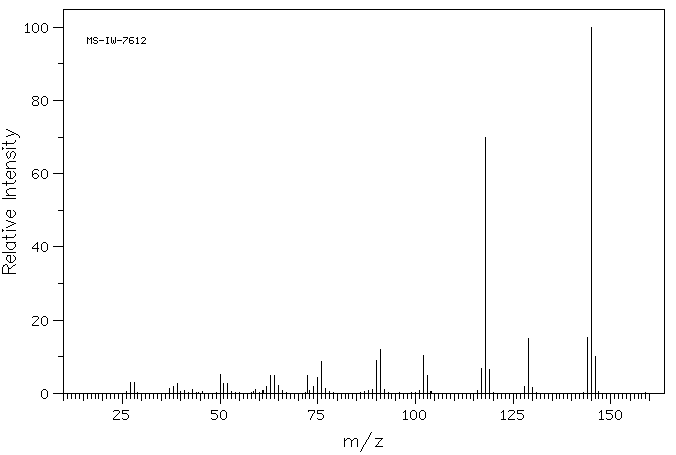
质谱MS
-
碳谱13CNMR
-
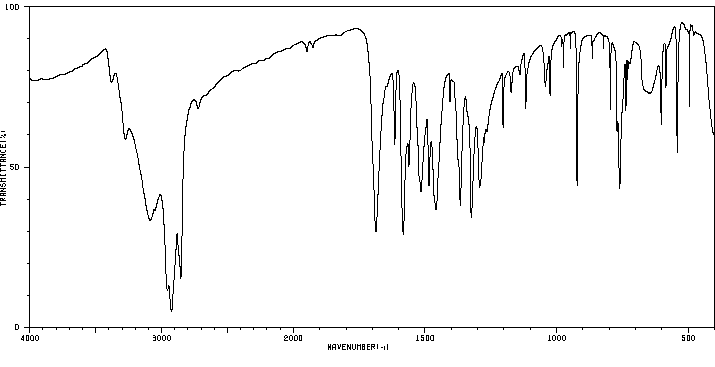
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮