4-苯基异噻唑 | 936-46-9
中文名称
4-苯基异噻唑
中文别名
——
英文名称
4-Phenyl-isothiazole
英文别名
4-Phenylisothiazole;4-phenyl-1,2-thiazole
CAS
936-46-9
化学式
C9H7NS
mdl
MFCD18451401
分子量
161.227
InChiKey
RAUUYMXHIUAZCW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
LogP:3.103 (est)
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:11
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:41.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-(4-bromophenyl)isothiazole 25392-19-2 C9H6BrNS 240.123 —— 4-isothiazol-4-yl-aniline 25392-18-1 C9H8N2S 176.242 —— 4-(4-chlorophenyl)isothiazole 25392-21-6 C9H6ClNS 195.672 5-氘代-4-苯基异噻唑 5-deuterio-4-phenyl-isothiazole 10514-31-5 C9H7NS 162.219 —— 2-isothiazol-4-yl-phenol 25392-16-9 C9H7NOS 177.227 —— 4-(4-nitro-phenyl)-isothiazole 25392-14-7 C9H6N2O2S 206.225 —— 4-isothiazol-4-yl-benzoic acid 25392-25-0 C10H7NO2S 205.237 —— 1-(4-isothiazol-4-yl-phenyl)-ethanone 25392-24-9 C11H9NOS 203.265 —— 5-bromo-4-(4-bromo-phenyl)-isothiazole 25392-20-5 C9H5Br2NS 319.019 —— 4-isothiazol-4-yl-benzenesulfonamide 25392-23-8 C9H8N2O2S2 240.307 —— 4-isothiazol-4-yl-benzenesulfonyl chloride 25392-22-7 C9H6ClNO2S2 259.737 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Phototransposition Chemistry of 4-Substituted Isothiazoles. The P4 Permutation Pathway摘要:Upon irradiation in the presence of a small quantity of base, 4-substituted isothiazoles undergo photocleavage to yield substituted cyanosulfides, which can be trapped as their benzyl thioether derivatives, and substituted isocyanosulfides. Both products are suggested to arise via initial photocleavage of the sulfur-nitrogen bond, resulting in the formation of a substituted beta-thioformylvinyl nitrene, which can rearrange to the observed cyanosulfide, or cyclize to an undetected thioformylazirine. Deprotonation of the azirine leads directly to the isocyanosulfide. The plight of the isocyanosulfide depends on the C-4 substituent. If the substituent is aromatic, the isocyanosulfide is reprotonated at the isocyanide carbon and spontaneously cyclizes to a 4-substituted thiazole, the observed transposition product. If the substituent is not aromatic, the isocyanosulfide is reprotonated at sulfur and the resulting species has a higher energy barrier to cyclization. In these cases, the isocyanosulfides can be observed spectroscopically and can be trapped as their N-formylaminobenzyl thioether derivatives.DOI:10.1021/jo980936e
-
作为产物:参考文献:名称:基于唑的吲哚胺 2,3-双加氧酶 1 (IDO1) 抑制剂摘要:血红素酶吲哚胺 2,3-双加氧酶 1 (IDO1) 通过催化色氨酸代谢犬尿氨酸途径中的限速步骤在免疫、神经元功能和衰老中发挥重要作用。已经开发了许多具有不同化学型的 IDO1 抑制剂,主要用于抗癌免疫治疗。由于血红素-配体相互作用的显着选择性和敏感性,直接血红素铁结合抑制剂的先导优化已被证明是困难的。在这里,我们提供了一组密切相关的小唑类化合物的实验数据,它们的抑制活性存在超过 4 个数量级的差异,范围从毫摩尔到纳摩尔水平。我们根据结构数据、分子动力学模拟和密度泛函理论计算来调查和合理化它们的活动。DOI:10.1021/acs.jmedchem.0c01968
文献信息
-
Access to 4-substituted isothiazoles through three-component cascade annulation and their application in C–H activation作者:Guoling Huang、Jian Li、Xiaoliang Ji、Lu Chen、Qiang Liu、Xiuwen Chen、Yubing Huang、Yibiao LiDOI:10.1039/d0cc01100b日期:——The use of potassium ethyl xanthate (EtOCS2k) as a sulfur atom donor enabled the transition-metal-free [3 + 1 + 1] cascade annulation of isopropene derivatives with NH4I in DMSO/H2O, affording various 4-substituted isothiazoles in moderate to good yields with good functional group compatibility. Furthermore, Pd and Ag-catalyzed C5-H-selective direct oxidation dimerization of 4-substituted isothiazoles
-
LIGANDS FOR ANTIBODY AND FC-FUSION PROTEIN PURIFICATION BY AFFINITY CHROMOTOGRAPHY IV申请人:GRAFFINITY PHARMACEUTICALS GMBH公开号:US20160009760A1公开(公告)日:2016-01-14The present invention relates to the use, for affinity purification of an antibody or an fragment of an antibody, of a ligand-substituted matrix comprising a support material and at least one ligand covalently bonded to the support material, the ligand being represented by formula (I) L-(Sp) v -Ar 1 —Am—Ar 2 (I) wherein L, SP, Ar 1 , AM, Ar 2 and v are defined herein.
-
Zur Oxidation von 1,2-Thiazolen: Ein einfacher Zugang zu 1,2-Thiazol-3(2H)-on-1,1-dioxiden作者:B�rbel Schulze、Gisela Kirsten、Sabine Kirrbach、Annette Rahm、Heinz HeimgartnerDOI:10.1002/hlca.19910740515日期:1991.8.7Oxidation of 1,2-Thiazoles; A Convenient Approach to 1,2-Thiazol-3(2H)-one 1,1-Dioxides1,2-噻唑的氧化; 1,2-Thiazol-3(2 H)-one 1,1-Dioxides的简便方法
-
The Reaction of some Isothiazolium Salts with Sulfur in Pyridine作者:G. E. Bachers、D. M. McKinnon、J. M. BuchshriberDOI:10.1139/v72-413日期:1972.8.15
A variety of N-methyl and N-phenylisothiazolium salts has been synthesized and treated with sulfur in boiling pyridine. The products have been examined by chromatography and their structures determined. While 5-unsubstituted isothiazolium salts appear to give the corresponding isothiazoline-5-thiones, 3-unsubstituted salts give either the corresponding isothiazoline-3-thiones if the nitrogen is alkyl substituted, or 1,2-dithiole-3-imines if the nitrogen is aryl substituted. N-Alkyl compounds also give dealkylated products, and dithiolethiones are also found. The initial stages in the reaction appear to involve deprotonation of the isothiazolium salt.
-
The reaction of 1,2-dithiolium salts with ammonia作者:R.A. Olofson、J.M. Landesberg、R.O. Berry、D. Leaver、W.A.H. Robertson、D.M. McKinnonDOI:10.1016/s0040-4020(01)82131-1日期:1966.14-Phenyl-1,2-dithiolium perchlorate reacts with ammonia in ethanol to yield 4-phenyl-isothiazole. The reaction is general for dithiolium salts. When unsymmetrical dithiolium salts are treated with ammonia, the major product and, under many conditions, the only product is the 5-substituted isothiazole. Of the four possible mechanisms for these reactions, an addition-elimination mechanism has been shown
表征谱图
-
氢谱1HNMR
-
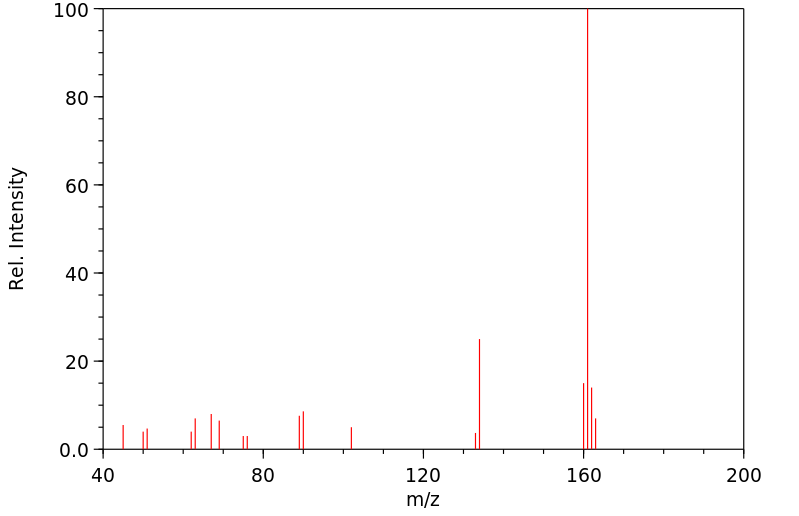
质谱MS
-
碳谱13CNMR
-
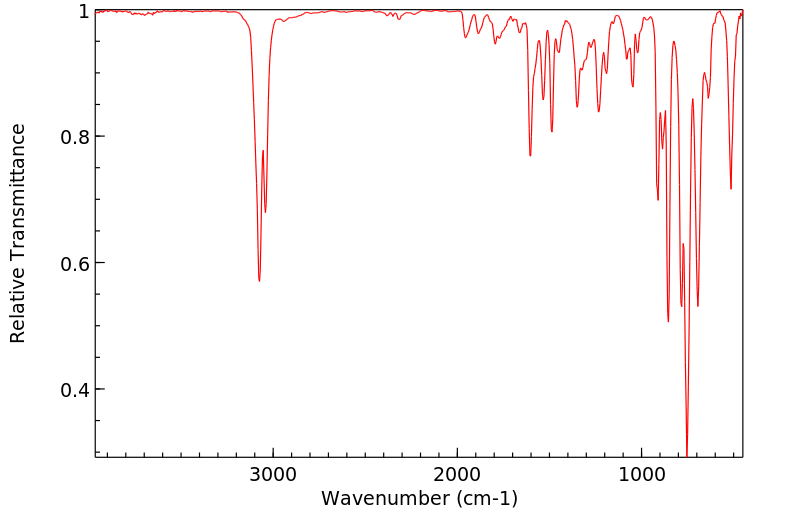
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫