3-methyl-1,5-diphenyl-4,5-dihydro-1H-pyrazole | 2515-46-0
中文名称
——
中文别名
——
英文名称
3-methyl-1,5-diphenyl-4,5-dihydro-1H-pyrazole
英文别名
3-Methyl-1,5-diphenyl-2-pyrazolin;1,5-diphenyl-3-methyl-2-pyrazoline;3-Methyl-1,5-diphenyl-pyrazolin;5-methyl-2,3-diphenyl-3,4-dihydropyrazole
CAS
2515-46-0
化学式
C16H16N2
mdl
——
分子量
236.316
InChiKey
FZTOIOYDPXSQDM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:18
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.19
-
拓扑面积:15.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2933199090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (4R,5S)-3,4-dimethyl-1,5-diphenyl-4,5-dihydro-1H-pyrazole 1314766-13-6 C17H18N2 250.343
反应信息
-
作为反应物:描述:3-methyl-1,5-diphenyl-4,5-dihydro-1H-pyrazole 在 air 、 pyrene and triazine-based porous organic polymer 作用下, 以 乙酸乙酯 为溶剂, 以71 %的产率得到1,5-二苯基-3-甲基-1H-吡唑参考文献:名称:Development of Porous Organic Polymers as Metal‐Free Photocatalysts for the Aromatization of N‐Heterocycles摘要:摘要 多孔有机聚合物(POPs),尤其是共价三嗪框架(CTFs),正被开发为下一代无金属异相光催化剂。然而,目前获得这些光活性持久性有机聚合物的许多合成路线都需要昂贵的单体并依赖贵金属催化剂,因此阻碍了它们的广泛应用。在这项研究中,我们通过路易斯酸催化聚合反应,从简单的非官能化芳香族结构单元合成了一系列持久性有机污染物。所获得的材料首次被用作 N-杂环芳香化的异相光催化剂。使用活性最高的材料 CTF-Pyr(由具有光活性的芘和三嗪分子组成),可以获得多种吡啶、二氢喹啉-5-酮、四氢吖啶-1,8-二酮和吡唑,收率极高(70-99%)。此外,这些反应都是在非常温和的条件下,利用空气在室温下进行的,突出了这些材料作为绿色转化催化剂的潜力。DOI:10.1002/cctc.202301205
-
作为产物:描述:参考文献:名称:Knorr; Duden, Chemische Berichte, 1893, vol. 26, p. 115摘要:DOI:
文献信息
-
Iron-catalyzed aerobic oxidative aromatization of 1,3,5-trisubstituted pyrazolines作者:Guddekoppa S. Ananthnag、Adithya Adhikari、Maravanji S. BalakrishnaDOI:10.1016/j.catcom.2013.09.002日期:2014.1A simple and high yielding method for the synthesis of tri-substituted pyrazoles via iron(III) catalyzed aerobic oxidative aromatization of pyrazolines has been reported. The process demonstrates a variety of functional group tolerance.
-
Unexpected ring opening of pyrazolines with activated alkynes: synthesis of 1<i>H</i>-pyrazole-4,5-dicarboxylates and chromenopyrazolecarboxylates作者:Sai Teja Kolla、Nageswara Rao Rayala、Balasubramanian Sridhar、China Raju BhimapakaDOI:10.1039/d1ob01727f日期:——prepared by reacting pyrazolines with activated alkynes under neat conditions without a catalyst. The products were formed via unexpected ring opening of pyrazolines with the elimination of styrene/ethylene. These types of transformations are unknown and the products formed were confirmed using their spectral/analytical data. In addition, the structures of compounds 5e and 5n were confirmed by single-crystal
-
Stereospecific Intramolecular C–H Amination of 1-Aza-2-azoniaallene Salts作者:Daniel A. Bercovici、Matthias BrewerDOI:10.1021/ja303054c日期:2012.6.20participate in intramolecular C-H amination reactions to provide pyrazoline products in good to excellent yield. This intramolecular amination occurs readily at both benzylic and tertiary aliphatic positions and proceeds at an enantioenriched chiral center without loss of enantiomeric excess. A competition reaction shows that insertion occurs more readily at an electron-rich benzylic position than an
-
Solid-Phase Synthesis of Pyrazolines and Isoxazolines with Sodium Benzenesulfinate as a Traceless Linker作者:Yu Chen、Yulin Lam、Yee-Hing LaiDOI:10.1021/ol0340888日期:2003.4.1text] The preparation of pyrazoline and isoxazoline derivatives with traceless solid-phase sulfone linker strategy is described. Key steps involved in the solid-phase synthetic procedure include (i) sulfinate S-alkylation, (ii) sulfone anion alkylation, (iii) gamma-hydroxy sulfone --> gamma-ketosulfone oxidation, and (iv) traceless product release via elimination-cyclization. A library of 12 pyrazolines
-
An efficient aerobic oxidative aromatization of Hantzsch 1,4-dihydropyridines and 1,3,5-trisubstituted pyrazolines作者:Bing Han、Zhengang Liu、Qiang Liu、Li Yang、Zhong-Li Liu、Wei YuDOI:10.1016/j.tet.2005.12.056日期:2006.34-Substituted Hantzsch 1,4-dihydropyridines and 1,3,5-trisubstituted pyrazolines were oxidized to the corresponding pyridines and pyrazoles, respectively, in high yields by molecular oxygen in the presence of catalytic amount of N-hydroxyphthalimide (NHPI) and Co(OAc)2 in acetonitrile at room temperature.
表征谱图
-
氢谱1HNMR
-
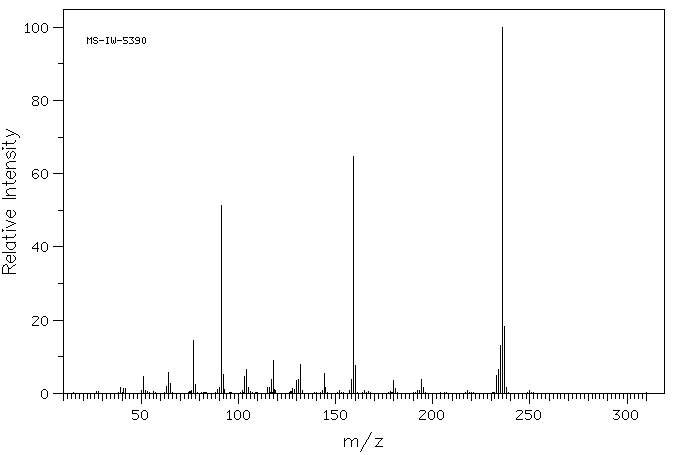
质谱MS
-
碳谱13CNMR
-
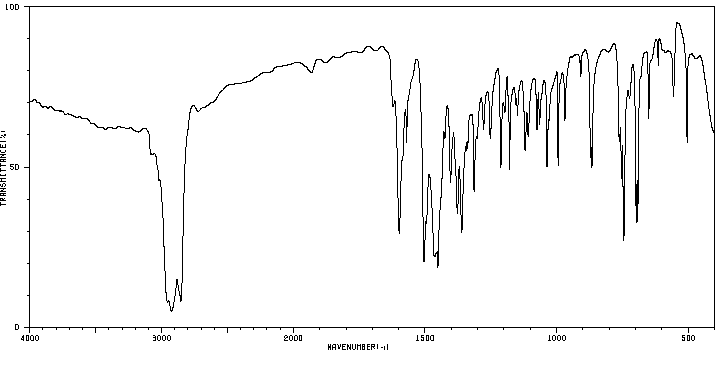
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)