3-hydroxy-3-(3,3-dimethyl-2-oxobutyl)-2-oxindole | 27302-71-2
中文名称
——
中文别名
——
英文名称
3-hydroxy-3-(3,3-dimethyl-2-oxobutyl)-2-oxindole
英文别名
3-(3,3-dimethyl-2-oxobutyl)-3-hydroxyindolin-2-one;3-(3,3-Dimethyl-2-ketobut-1-yl)-hydroxyoxindol;3-(3,3-dimethyl-2-oxo-butyl)-3-hydroxy-1,3-dihydro-indol-2-one;3-hydroxy-3-(3,3-dimethyl-2-oxo-butyl)-indolin-2-one;3-Hydroxy-3-(3,3-dimethyl-2-oxo-butyl)-indolin-2-on;3-(3,3-Dimethyl-2-oxobutyl)-3-hydroxy-2-indolinone;3-(3,3-dimethyl-2-oxobutyl)-3-hydroxy-1H-indol-2-one
CAS
27302-71-2
化学式
C14H17NO3
mdl
——
分子量
247.294
InChiKey
JWDUMWNFXPKAJN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:18
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.43
-
拓扑面积:66.4
-
氢给体数:2
-
氢受体数:3
反应信息
-
作为反应物:描述:3-hydroxy-3-(3,3-dimethyl-2-oxobutyl)-2-oxindole 在 硼烷四氢呋喃络合物 作用下, 以 四氢呋喃 为溶剂, 以82%的产率得到3-(3',3'-Dimethyl-2-hydroxybutyl)indol参考文献:名称:A versatile synthetic methodology for the synthesis of tryptophols摘要:Tryptophols have been obtained in high yields by the reduction of 3-substituted-dioxindoles (obtained by the aldol condensation reaction of ketones with isatins or by a modified Knovenagel malonate condensation) using a borane tetrahydrofuran complex. The reported methodology offers distinct advantages over existing methods for the synthesis of these compounds, including consistently greater yields, diastereoselective syntheses and the possibility for the synthesis of a wide range of structurally different tryptophols. The reduction reaction was found to proceed via an intermediate 1,3-diol-oxindole, which was obtained diastereoselectively and, which was subsequently reduced to the corresponding tryptophol. (C) 2002 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4020(02)01048-7
-
作为产物:描述:4,4-dimethyl-3-oxopentanoic acid 、 靛红 以 乙酸乙酯 为溶剂, 反应 12.0h, 以83%的产率得到3-hydroxy-3-(3,3-dimethyl-2-oxobutyl)-2-oxindole参考文献:名称:分子筛介导的β-酮酸脱羧曼尼希和羟醛反应摘要:报道了分子筛介导的β-酮酸与磺酰基亚胺的曼尼希脱羧反应。该方案可有效制备合成上有用的β-氨基酮。还描述了类似的分子筛促进的β-酮酸和靛红之间的脱羧醛醇缩合反应,其以优异的产率提供了生物活性的3-取代的-3-羟基-吲哚。DOI:10.1016/j.tetlet.2013.06.030
文献信息
-
A New Mild Method for Synthesis of Marine Alkaloid Fascaplysin and Its Therapeutically Promising Derivatives作者:Oleg A. Tryapkin、Alexey V. Kantemirov、Sergey A. Dyshlovoy、Vladimir S. Prassolov、Pavel V. Spirin、Gunhild von Amsberg、Maria A. Sidorova、Maxim E. ZhidkovDOI:10.3390/md21080424日期:——addition, the number of methods for the syntheses of its derivatives is limited. In the current study, we report a new two-step method of synthesis of fascaplysin derivatives based on low temperature UV quaternization for the synthesis of thermolabile 9-benzyloxyfascaplysin and 6-tert-butylfascaplysin. 9-Benzyloxyfascaplysin was used as the starting compound to obtain 9-hydroxyfascaplysin. HoweverFascaplysin 是一种海洋生物碱,由于其多样化且有效的生物活性而被认为是主要候选药物。作为一种抗癌剂,fascaplysin 具有巨大的潜力,因为这种生物碱在癌细胞中影响多个靶点,包括抑制细胞周期蛋白依赖性激酶 4 (CDK4) 和诱导内在细胞凋亡。同时,其结构优化的研究也因其较高的毒性(主要是由DNA嵌入引起)而受到阻碍。此外,其衍生物的合成方法数量有限。在目前的研究中,我们报道了一种基于低温紫外季铵化的新型两步法 fascaplysin 衍生物合成方法,用于合成不耐热的 9-benzyloxyfascaplysin 和 6-tert-丁基 fascaplysin。使用9-苄氧基fascaplysin作为起始化合物以获得9-羟基fascaplysin。然而,发现后者在化学上高度不稳定。与 fascaplysin 相比,6-tert-Butylfascaplysin 的 DNA 嵌
-
Pietra; Tacconi, Farmaco, Edizione Scientifica, 1958, vol. 13, p. 893,907作者:Pietra、TacconiDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
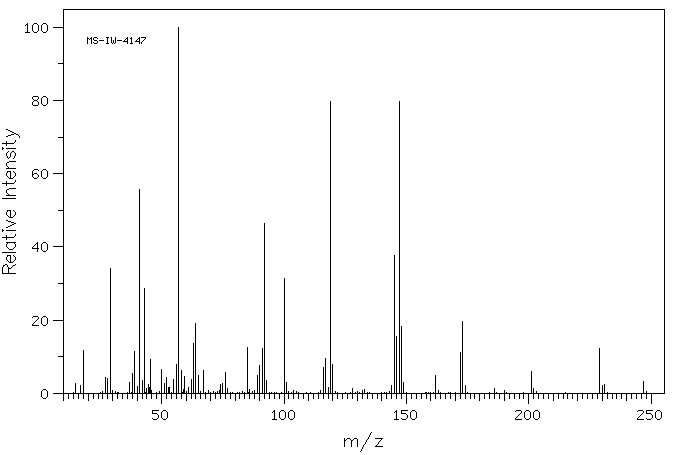
质谱MS
-
碳谱13CNMR
-
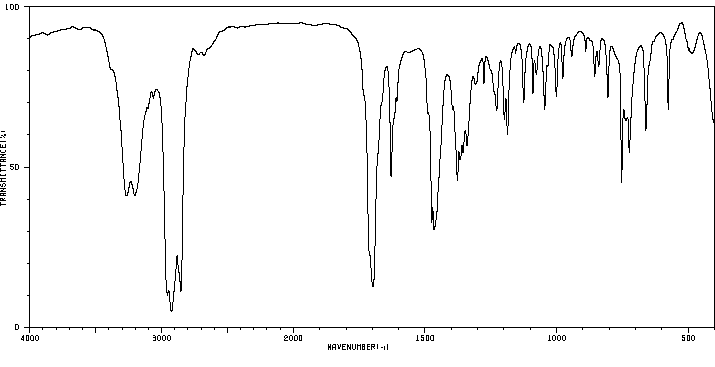
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3