phenethyl 3-phenylpropanoate | 28049-10-7
中文名称
——
中文别名
——
英文名称
phenethyl 3-phenylpropanoate
英文别名
2-phenylethyl 3-phenylpropanoate;phenethyl 3-phenylpropionate;3-Phenylpropionic acid, 2-phenylethyl ester
CAS
28049-10-7
化学式
C17H18O2
mdl
——
分子量
254.329
InChiKey
QQNDZUIFXZRWFO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:171 °C(Press: 11 Torr)
-
密度:1.077±0.06 g/cm3(Predicted)
-
保留指数:2015
计算性质
-
辛醇/水分配系数(LogP):4.3
-
重原子数:19
-
可旋转键数:7
-
环数:2.0
-
sp3杂化的碳原子比例:0.24
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:phenethyl 3-phenylpropanoate 在 polymethylhydrosiloxane 作用下, 以 甲苯 为溶剂, 以92%的产率得到3-phenylpropyl 2-phenylethyl ether参考文献:名称:脂肪族羧酸和醇通过酯的铟催化脱氧一锅法顺序合成醚摘要:我们开发了一种广泛适用的直接醚化方法,通过一锅反应中形成的瞬态酯的铟催化脱氧从羧酸和醇中进行醚化。这种简单的催化还原系统似乎对包括烯烃、卤素、硝基和杂环基团在内的几种官能团具有显着的耐受性。DOI:10.1002/ejoc.201200552
-
作为产物:描述:参考文献:名称:使用两亲整体-SO3H 树脂对羧酸与醇或硫醇进行酯化或硫酯化摘要:我们开发了一种使用带有磺酸部分的两亲性整体树脂作为阳离子交换功能(整体-SO 3 H)将羧酸与醇酯化的方法。Monolith-SO 3 H 在 60–80 °C 下,在甲苯中有效地催化芳香族和脂肪族羧酸与各种伯醇和仲醇(1.5–5.0 当量)的酯化反应,无需去除反应过程中产生的水。Monolith-SO 3的两亲性H 由于其吸水能力而促进脱水。该反应也适用于硫酯化,尽管需要在 120 °C 下加热,但仅使用 2.0 当量的硫醇在甲苯中即可以优异的收率获得相应的硫酯。此外,整体式-SO 3 H 可通过简单的过滤从反应混合物中分离出来,并在不降低催化活性的情况下重复使用至少五次。DOI:10.1246/bcsj.20210266
文献信息
-
Manganese-catalyzed homogeneous hydrogenation of ketones and conjugate reduction of α,β-unsaturated carboxylic acid derivatives: A chemoselective, robust, and phosphine-free in situ-protocol作者:Thomas Vielhaber、Christoph TopfDOI:10.1016/j.apcata.2021.118280日期:2021.8glove-box-free catalytic protocol for the manganese-catalyzed hydrogenation of ketones and conjugated CCbonds of esters and nitriles. The respective catalyst is readily assembled in situ from the privileged [Mn(CO)5Br] precursor and cheap 2-picolylamine. The catalytic transformations were performed in the presence of t-BuOK whereby the corresponding hydrogenation products were obtained in good to excellent yields
-
Syntheses and reactivities of non-symmetrical “active ester” bi-dentate cross-linking reagents having a phthalimidoyl and acid chloride, 2-benzothiazole, or 1-benzotriazole group作者:Md. Chanmiya Sheikh、Shunsuke Takagi、Mebumi Sakai、Tasuya Mori、Naoto Hayashi、Tetsuo Fujie、Shin Ono、Toshiaki Yoshimura、Hiroyuki MoritaDOI:10.1039/c0ob00671h日期:——active ester” bi-dentate cross-linking reagents having an acid chloride, 2-benzothiazole, or 1-benzotriazole group (i.e., 9, 15, and 16) on the basis of the reactivity study of the “active ester” model compounds, 11–14, toward the various nucleophiles and examined their reaction selectivity towards the same nucleophiles. Then, we applied for the modification of cholesterol at the more reactive site of
-
Broadly Applicable Ytterbium-Catalyzed Esterification, Hydrolysis, and Amidation of Imides作者:Céline Guissart、Andre Barros、Luis Rosa Barata、Gwilherm EvanoDOI:10.1021/acs.orglett.8b01896日期:2018.9.7An efficient, broadly applicable, operationally simple, and divergent process for the transformation of imides into a range of carboxylic acid derivatives under mild conditions is reported. By simply using catalytic amounts of ytterbium(III) triflate as a Lewis acid promoter in the presence of alcohols, water, amines, or N,O-dimethylhydroxylamine, a broad range of imides is smoothly and readily converted
-
Development of a triazinedione-based dehydrative condensing reagent containing 4-(dimethylamino)pyridine as an acyl transfer catalyst作者:Jie Liu、Hikaru Fujita、Masanori Kitamura、Daichi Shimada、Munetaka KunishimaDOI:10.1039/d1ob00450f日期:——the operationally simple dehydrative condensation of carboxylic acids. This reagent comprises an ATD core and DMAP as the leaving group, which is liberated into the reaction system to accelerate acyl transfer reactions. Upon adding ATD-DMAP to a mixture of carboxylic acids and alcohols in the presence of an amine base, the corresponding esters were formed rapidly at room temperature. Moreover, dehydrative
-
Development of triazine-based esterifying reagents containing pyridines as a nucleophilic catalyst作者:Kohei Yamada、Jie Liu、Munetaka KunishimaDOI:10.1039/c8ob01660g日期:——as a nucleophilic catalyst. 1-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-3,5-lutidinium chloride (DMT-3,5-LUT) was found to exhibit a superior reactivity for the dehydrating condensation reaction between carboxylic acids and alcohols. The reaction of DMT-3,5-LUT with carboxylic acids produces intermediacy of acyloxytriazines, which is known to exhibit moderate reactivity toward alcohols, with concomitant liberation
表征谱图
-
氢谱1HNMR
-
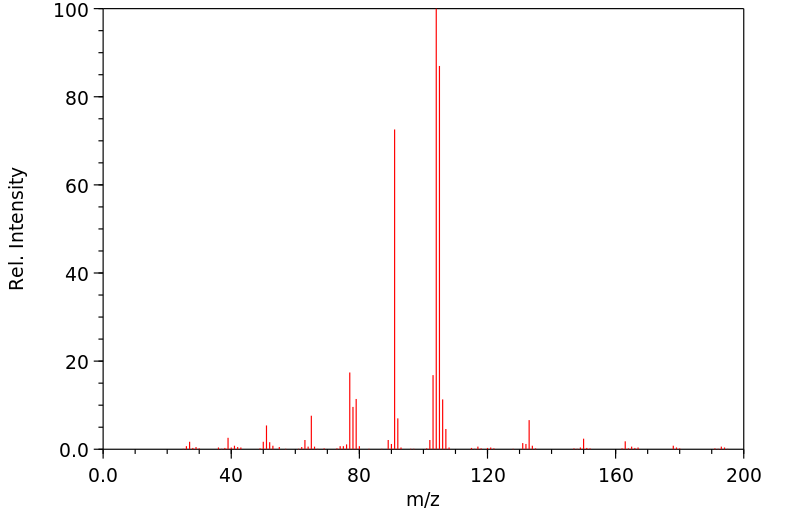
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯