(E)-4-(2-nitro-but-1-enyl)benzonitrile | 952316-10-8
中文名称
——
中文别名
——
英文名称
(E)-4-(2-nitro-but-1-enyl)benzonitrile
英文别名
Rjyslzoafuspfu-yrnvussqsa-;4-[(E)-2-nitrobut-1-enyl]benzonitrile
CAS
952316-10-8
化学式
C11H10N2O2
mdl
——
分子量
202.213
InChiKey
RJYSLZOAFUSPFU-YRNVUSSQSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:109-110 °C
-
沸点:363.9±25.0 °C(predicted)
-
密度:1.18±0.1 g/cm3(Temp: 20 °C; Press: 760 Torr)(predicted)
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:15
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.18
-
拓扑面积:69.6
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为反应物:描述:(E)-4-(2-nitro-but-1-enyl)benzonitrile 、 2-(吡啶-1-基)乙腈溴化物 在 silver carbonate 作用下, 以 四氢呋喃 为溶剂, 反应 2.0h, 以70%的产率得到2-(4-cyanophenyl)-1-ethylindolizine-3-carbonitrile参考文献:名称:多取代吲哚嗪的模块化合成摘要:吡啶与氰醇三氟甲磺酸酯或 α-卤腈的 N-烷基化提供 1-(1-氰基烷基)吡啶鎓盐,该盐可以在碱性条件下与硝基烯烃反应以提供多取代的茚茚。总的来说,吲哚嗪核可以由一个吡啶、两个醛和一个硝基烷烃构成,并且产物中没有残留的不需要的官能团。当溴乙腈用于 N-烷基化时,得到 indolizine-3-carbonitriles。吡啶组分可以被其他吖嗪替代,从而产生相关的杂环系统。DOI:10.1002/ejoc.201200424
-
作为产物:参考文献:名称:Modular One-Pot Synthesis of Tetrasubstituted Pyrroles from α-(Alkylideneamino)nitriles摘要:[GRAPHICS]2,3,4,5-Tetrasubstituted pyrroles have been prepared with high regioselectivity by a formal cycloaddition of alpha-(alkylideneamino)nitriles and nitroolefins followed by elimination of HCN and HNO2. The reaction allows the convergent construction of the pyrrole ring in four steps from a nitroalkane and three aldehydes.DOI:10.1021/jo070426x
文献信息
-
Substrate selective synthesis of pyrazolo[1,5-a]pyridines through [3 + 2] cycloaddition of N-aminopyridines and β-nitro styrenes作者:Chitrakar Ravi、Darapaneni Chandra Mohan、N. Naresh Kumar Reddy、Subbarayappa AdimurthyDOI:10.1039/c5ra06707c日期:——
Synthesis of 2 or 3-(hetero)aryl pyrazolo[1,5-
a ]pyridines through [3 + 2] cycloaddition ofN -aminopyridine with β-nitrostyrenes followed byin situ denitration under metal-free and mild conditions are described.
表征谱图
-
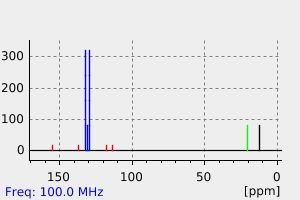
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫