S-hexyl octanethioate | 55590-85-7
中文名称
——
中文别名
——
英文名称
S-hexyl octanethioate
英文别名
hexyl thiooctanoic acid ester;n-Heptancarbonsaeure-thio-n-hexylester;S-hexyl thiooctanoate;Octanethioic acid, hexyl ester
CAS
55590-85-7
化学式
C14H28OS
mdl
——
分子量
244.442
InChiKey
ARKGQSZBBOGCSX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):6
-
重原子数:16
-
可旋转键数:12
-
环数:0.0
-
sp3杂化的碳原子比例:0.93
-
拓扑面积:42.4
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:3-巯丙基三乙氧基硅烷 、 S-hexyl octanethioate 在 乙醇 、 sodium ethanolate 作用下, 80.0~120.0 ℃ 、18.67 kPa 条件下, 反应 8.0h, 生成 3-辛酰基硫代丙基三乙氧基硅烷参考文献:名称:[EN] METHOD FOR THE MANUFACTURE OF ALKOXYSILYL-CONTAINING THIOCARBOXYLIC ACID ESTERS
[FR] PROCÉDÉ DE FABRICATION D'ESTERS D'ACIDE THIOCARBOXYLIQUE CONTENANT DE L'ALKOXYSILYLE摘要:本文提供了一种制备含烷氧硅基硫代羧酸酯的方法,包括将硫酯与巯基功能烷氧硅烷和/或碱金属盐、碱土金属盐、烷氧硅基硫醇酸三取代铵盐反应,使用经济实惠且易得的试剂,避免使用光气或氯化硫酰试剂,并产生可循环利用的副产物。公开号:WO2020223068A1 -
作为产物:描述:2-氯-3-甲基苯并噻唑鎓三氟甲烷磺酸盐 以92%的产率得到参考文献:名称:SOUTO-BACHILLER F.; BATES G. S.; MASAMUNE S., J. CHEM. SOC. CHEM. COMMUNS
, 1976, NO 18, 719-720 摘要:DOI:
文献信息
-
One for Many: A Universal Reagent for Acylation Processes作者:Hyun Kyung Moon、Gi Hyeon Sung、Bo Ram Kim、Jong Keun Park、Yong-Jin Yoon、Hyo Jae YoonDOI:10.1002/adsc.201501177日期:2016.6.2This work describes acylation reactions facilitated by a type of heterocycle‐based acyl transfer agent, 2‐acyloxypyridazinone. Reactions of 2‐acyloxypyridazinone with carboxylic acids yield mixed carbonic anhydride intermediates, which are reactive and could be coupled with a wide range of substrates including acids, amines, alcohols, and thiols. The wide substrate scope, ease of operation (no additive
-
2-Chloro-N-methylbenzothiazolium trifluoromethanesulphonate: an efficient condensing agent for the preparation of thiol esters and amides作者:Fernando Souto-Bachiller、Gordon S. Bates、Satoru MasamuneDOI:10.1039/c39760000719日期:——2-Chloro-N-methylbenzothiazolium trifluoromethanesulphonate is readily prepared and handled, and effects the formation of thiol esters and amides in excellent yields under mild non-acidic conditions.
-
Lipase chemoselectivity towards alcohol and thiol acyl acceptors in a transacylation reaction作者:Cecilia Hedfors、Karl Hult、Mats MartinelleDOI:10.1016/j.molcatb.2010.04.005日期:2010.9The lipase chemoselectivity towards an alcohol and a thiol was investigated for the two lipases Candida antarctica lipase B (CalB) and Rhizomucor miehei lipase (Rml). Hexanol and hexanethiol were used as acyl acceptors in a transacylation reaction with ethyl octanoate in cyclohexane. CalB showed the highest chemoselectivity ratio (k(cat)/K-M)(OH)/(k(cat)/K-M)(SH), of 88,000 while the ratio for Rml was 1200. That could be compared with the ratio, k(OH)/k(SH), of 120 for the non-catalyzed reaction. Thus, the enzyme contribution to the chemoselectivity between hexanol and hexanethiol was 730 for CalB and 10 for Rml. High K-M values displayed towards hexanethiol (above 1.8 M) were the largest contribution to the selectivity. No saturation was achieved. The K-M values were more than two orders of magnitude higher than those of hexanol. (C) 2010 Elsevier B.V. All rights reserved.
表征谱图
-
氢谱1HNMR
-
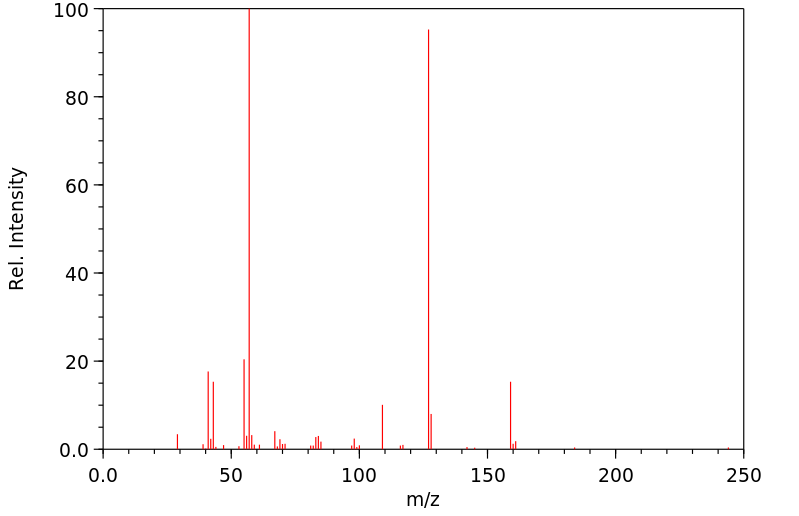
质谱MS
-
碳谱13CNMR
-
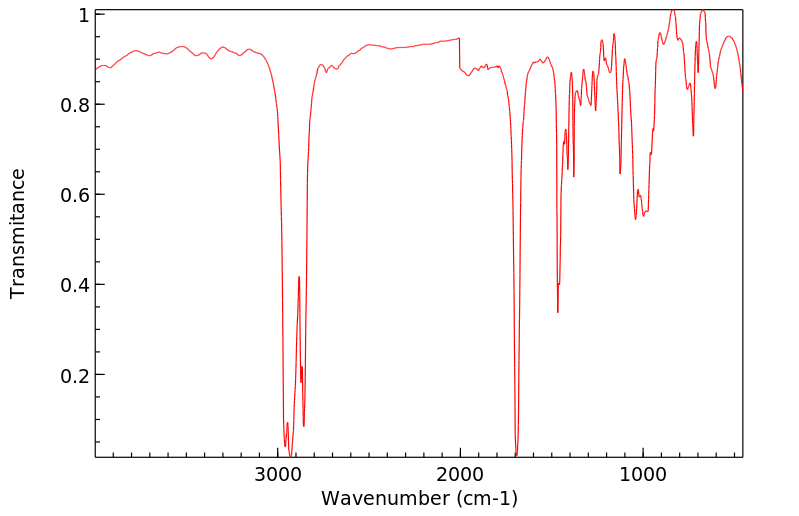
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯