curcumin | 147556-16-9
中文名称
——
中文别名
——
英文名称
curcumin
英文别名
(1E,4Z,6E)-5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,4,6-trien-3-one;diferuloylmethane
CAS
147556-16-9
化学式
C21H20O6
mdl
——
分子量
368.386
InChiKey
ZIUSSTSXXLLKKK-KOBPDPAPSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:181-182 °C(Solv: acetone (67-64-1))
-
沸点:593.2±50.0 °C(Predicted)
-
密度:1.307±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:27
-
可旋转键数:7
-
环数:2.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:96.2
-
氢给体数:3
-
氢受体数:6
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 姜黄素 curcumin 458-37-7 C21H20O6 368.386 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 ASC-J9; 二甲基姜黄素 O,O-dimethyl-curcumin 52328-98-0 C23H24O6 396.44 —— (1E,4Z,6E)-1-(3,4-dimethoxyphenyl)-5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)hepta-1,4,6-trien-3-one —— C22H22O6 382.413 —— (1E,4Z,6E)-1,7-bis(4-ethoxy-3-methoxyphenyl)-5-hydroxyhepta-1,4,6-trien-3-one —— C25H28O6 424.494 —— (1E,4Z,6E)-1-(4-butoxy-3-methoxyphenyl)-5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)hepta-1,4,6-trien-3-one —— C25H28O6 424.494 —— (1E,4Z,6E)-1,7-bis(4-(benzyloxy)-3-methoxyphenyl)-5-hydroxyhepta-1,4,6-trien-3-one —— C35H32O6 548.635 —— (1E,4Z,6E)-5-amino-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,4,6-trien-3-one 1010805-60-3 C21H21NO5 367.401 —— (1E,4Z,6E)-1,7-bis(2,5-dimethoxyphenyl)-5-hydroxyhepta-1,4,6-trien-3-one 1400942-75-7 C23H24O6 396.44 二-(叔-丁基-二甲基硅烷基)姜黄素 (1E,4Z,6E)-1,7-bis(4-((tert-butyldimethylsilyl)oxy)-3-methoxyphenyl)-5-hydroxyhepta-1,4,6-trien-3-one 1134639-23-8 C33H48O6Si2 596.912 —— (1E,4Z,6E)-1-(4-((tert-butyldimethylsilyl)oxy)-3-methoxyphenyl)-5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)hepta-1,4,6-trien-3-one —— C27H34O6Si 482.649 —— (1E,4Z,6E)-5-(ethylamino)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,4,6-trien-3-one 1010805-64-7 C23H25NO5 395.455 —— 5-hydroxy-1,7-bis-[3-methoxy-4-(tetrahydropyran-2-yloxy)-phenyl]-hepta-1,4,6-trien-3-one 884506-67-6 C31H36O8 536.622 —— (1E,4Z,6E)-1,7-bis(2,4-dimethoxyphenyl)-5-hydroxyhepta-1,4,6-trien-3-one —— C23H24O6 396.44 —— (1E,4Z,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-5-(propan-2-ylamino)hepta-1,4,6-trien-3-one 1010805-62-5 C24H27NO5 409.482 —— (1E,4Z,6E)-5-hydroxy-1,7-bis(2,4,6-trimethoxyphenyl)hepta-1,4,6-trien-3-one 1400942-76-8 C25H28O8 456.493 —— (1E,4Z,6E)-5-(benzylamino)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,4,6-trien-3-one 1010805-61-4 C28H27NO5 457.526 —— (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-4-(3-methoxybenzylidene)hepta-1,6-diene-3,5-dione 1257441-68-1 C29H26O7 486.521 —— (1E,6E)-4-(3,4-dimethoxybenzylidene)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione 1257441-62-5 C30H28O8 516.548 —— 4,4'-((1E,6E)-4-(hydroxymethylene)-3,5-dioxohepta-1,6-diene-1,7-diyl)bis(2-methoxy-4,1-phenylene) diacetate 1257441-69-2 C26H24O9 480.471 —— (1E,4Z,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-5-(1-phenylethylamino)hepta-1,4,6-trien-3-one 1010805-63-6 C29H29NO5 471.553 —— (1E,6E)-1,7-bis(2,5-dimethoxyphenyl)-4-(4-hydroxy-3-methoxybenzylidene)hepta-1,6-diene-3,5-dione 1400942-88-2 C31H30O8 530.574 —— (1E,6E)-1,7-bis(2,5-dimethoxyphenyl)-4-(3-hydroxy-4-methoxybenzylidene)hepta-1,6-diene-3,5-dione 1400942-93-9 C31H30O8 530.574 —— (1E,6E)-4-(3,4-dimethoxybenzylidene)-1,7-bis(2,5-dimethoxyphenyl)hepta-1,6-diene-3,5-dione 1400942-89-3 C32H32O8 544.601 —— (1E,6E)-4-(2,5-dimethoxybenzylidene)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione 1257441-56-7 C30H28O8 516.548 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Antitumor effects of curcumin and structurally β-diketone modified analogs on multidrug resistant cancer cells摘要:Using concepts of bioisostery a series of curcumin analogs were synthesized: the diketonic system of the compound was elaborated into enamitiones, oximes, and the isoxazole heterocycle. The cell growth inhibitory and apoptosis inducing effects of the new analogs were evaluated by in vitro assays in the hepatocellular carcinoma HA22T/VGH cells, as well as in the MCF-7 breast cancer cell line and in its multidrug resistant (MDR) variant MCF-7R. Increased antitumor activity on all cell lines was found with the isoxazole analog and especially with the benzyl oxime derivative; in the HA22T/VGH cell model, the latter compound inhibited constitutive NF-kappa B activation. (c) 2007 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2007.11.021
-
作为产物:参考文献:名称:Synthesis and Identification of New 4-Arylidene Curcumin Analogues as Potential Anticancer Agents Targeting Nuclear Factor-κB Signaling Pathway摘要:A series of curcumin analogues including new 4-arylidene curcumin analogues (4-arylidene-1,7-bisarylhepta-1,6-diene-3,5-diones) were synthesized. Cell growth inhibition assays revealed that most 4-arylidene curcumin analogues can effectively decrease the growth of a panel of lung cancer cells at submicromolar and low micromolar concentrations. High content analysis technology coupled with biochemical studies showed that this new class of 4-arylidene curcumin analogues exhibits significantly improved NF-kappa B inhibition activity over the parent compound curcumin, at least in part by inhibiting I kappa B phosphorylation and degradation via IKK blockage; selected 4-arylidene curcumin analogues also reduced the tumorigenic potential of cancer cells in a clonogenic assay.DOI:10.1021/jm1004545
文献信息
-
[EN] ENCAPSULATES<br/>[FR] PRODUITS ENCAPSULÉS申请人:PROCTER & GAMBLE公开号:WO2013022949A1公开(公告)日:2013-02-14The present application relates to encapsulates, compositions, products comprising such encapsulates, and processes for making and using such encapsulates. Such encapsulates comprise a core comprising a perfume and a shell that encapsulates said core, such encapsulates may optionally comprise a parametric balancing agent, such shell comprising one or more azobenzene moieties.
-
Synthesis, pharmacological profile and 2D-QSAR studies of curcumin-amino acid conjugates as potential drug candidates作者:Siva S. Panda、Adel S. Girgis、Sean J. Thomas、Jason E. Capito、Riham F. George、Asmaa Salman、May A. El-Manawaty、Ahmed SamirDOI:10.1016/j.ejmech.2020.112293日期:2020.6good yields utilizing an optimized reaction condition. We explored the effect of different amino acids and protecting groups on biological activities of curcumin. The conjugates were screened for anti-inflammatory, analgesic and antimicrobial properties. Some of the conjugates showed promising biological observations with a potency comparable with the standard references. The variations in biological properties
-
[EN] SUBSTITUTED METHYLFORMYL REAGENTS AND METHOD OF USING SAME TO MODIFY PHYSICOCHEMICAL AND/OR PHARMACOKINETIC PROPERTIES OF COMPOUNDS<br/>[FR] RÉACTIFS DE MÉTHYLFORMYLE SUBSTITUÉ ET PROCÉDÉ D'UTILISATION DE CEUX-CI POUR MODIFIER DES PROPRIÉTÉS PHYSICOCHIMIQUES ET/OU PHARMACOCINÉTIQUES DE COMPOSÉS申请人:SPHAERA PHARMA PRIVATE LTD公开号:WO2012137225A1公开(公告)日:2012-10-11The present invention relates to the synthesis and application of novel chiral/ achiral substituted methyl formyl reagents to modify pharmaceutical agents and/or biologically active substances to modify the physicochemical, biological and/or pharmacokinetic properties of the resulting compounds from the unmodified original agent.
-
Aryl fluorosulfate analogues as potent antimicrobial agents: SAR, cytotoxicity and docking studies作者:Lekkala Ravindar、S.N.A. Bukhari、K.P. Rakesh、H.M. Manukumar、H.K. Vivek、N. Mallesha、Zhi-Zhong Xie、Hua-Li QinDOI:10.1016/j.bioorg.2018.08.001日期:2018.12that the antimicrobial activity depends upon the presence of –OSO2F group and slender effect of different substituent’s on the phenyl rings. The electron donating (OCH3) groups in analogs increase the antibacterial activity, and interestingly the electron withdrawing (Cl, NO2, F and Br) groups increase the antifungal activity (except compound 35, 36 and 37). The mechanism of potent compounds showed membrane一系列芳基氟代类似物(的1 - 37)的合成和测试体外抗细菌和抗真菌研究,并通过对接研究验证。化合物9、12、14、19、25、26、35、36和37对测试的细菌菌株显示出优异的抗菌效力,而化合物2、4、5、15、35、36和37被发现具有更好的抗真菌活性分别与标准抗生素庆大霉素和酮康唑相比,可抵抗测试的真菌菌株。在所有的合成37点的类似物,化合物25,26,35,3637和37对金黄色葡萄球菌显示出优异的抗生物膜特性。结构-活性关系(SAR)表明,抗菌活性取决于–OSO 2 F基团的存在以及不同取代基对苯环的细长作用。类似物中的给电子基团(OCH 3)增加了抗菌活性,有趣的是,吸电子基团(Cl,NO 2,F和Br)增加了抗真菌活性(化合物35、36和37除外)。SEM证实了强效化合物的作用机理表明其对细菌的膜损伤。化合物35,36和在分子对接研究中,有37个滑翔g得分最高,并证明了其杀生物性质。
-
Reducing Platelet Activation, Aggregation and Platelet-Stimulated Thrombosis or Blood Coagulation by Reducing Mitochondrial Respiration申请人:Collman James P.公开号:US20110301180A1公开(公告)日:2011-12-08It has been discovered that inhibiting mitochondrial respiration in platelets reduces platelet activation or platelet aggregation. Certain heterocyclic compounds significantly reduced one or more platelet functions including clumping, sticking or platelet-stimulated clotting. Thus diseases or disorders mediated by inappropriately high levels of platelet activation or platelet aggregation can be treated by administering a therapeutically effective amount of a heterocyclic compound or nonheterocyclic mitochondrial inhibitor that significantly reduces one or more platelet functions including clumping, sticking or platelet-stimulated clotting, preferably in a reversible manner.
表征谱图
-
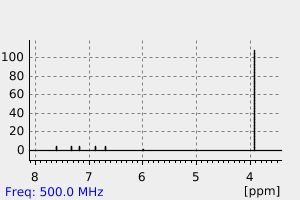
氢谱1HNMR
-
质谱MS
-
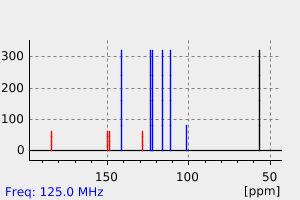
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(11aR)-3,7-双(3,5-二甲基苯基)-10,11,12,13-四氢-5-羟基-5-氧化物-二茚基[7,1-de:1'',7''-fg][1,3,2]二氧杂膦酸
龙血素C
顺-1,7-二苯基-1-庚烯基-5-醇
那洛西芬
赤杨酮
赤杨二醇
血竭素
蒙桑酮C
萘-2,7-二磺基酸,钠盐
苯酚,4-(1,3-二苯基丁基)-2-(1-苯基乙基)-
苯甲酸,2-[[2-[(2-羧基苯基)氨基]-5-(三氟甲基)苯基]氨基]-5-[[[(4-羟基-3-甲氧苯基)甲基]氨基]甲基]-
苯基-[4-(2-苯基乙炔基)苯基]甲酮
苯基-[2-[3-(三氟甲基)苯基]苯基]甲酮
苯基-[2-(2-苯基苯基)苯基]甲酮
苯基-(3-苯基萘-2-基)甲酮
苯基-(2-苯基环己基)甲酮
苯,[(二甲基苯基)甲基]甲基[(甲基苯基)甲基]-
苯,1,3-二[1-甲基-1-[4-(4-硝基苯氧基)苯基]乙基]-
脱甲氧姜黄
紫外吸收剂 234
粗糠柴苦素
硫酸姜黄素
矮紫玉盘素
益智醇
白桦林烯酮;1,7-双(4-羟基苯基)-4-庚烯-3-酮
甲酮,苯基(1,6,7,8-四氢-1-甲基-5-苯基环戊二烯并[g]吲哚-3-基)-
甲酮,[3-(4-甲氧苯基)-1-苯基-9H-芴-4-基]苯基-
甲酮,(4-氯苯基)[1-(4-氯苯基)-3-苯基-9H-芴-4-基]-
环香草酮
溴敌隆
波森
桤木酮
桑根酮D
杨梅醇
杨梅酮
杨梅联苯环庚醇-15-葡糖苷
替拉那韦
替吡法尼(S型对映体)
替吡法尼
曲沃昔芬
姜黄素葡糖苷酸
姜黄素beta-D-葡糖苷酸
姜黄素4,4'-二乙酸酯
姜黄素-d6
姜黄素
姜烯酮 A
奈帕芬胺杂质D
四甲基姜黄素
四氢脱甲氧基二阿魏酰甲烷
四氢姜黄素二乙酸酯