3-methyl-2-[3-oxo-4-(2-phenyl-acetylamino)-3H-isothiazol-2-yl]-but-3-enoic acid methyl ester | 34104-24-0
中文名称
——
中文别名
——
英文名称
3-methyl-2-[3-oxo-4-(2-phenyl-acetylamino)-3H-isothiazol-2-yl]-but-3-enoic acid methyl ester
英文别名
Methyl 3-methyl-2-[3-oxo-4-[(2-phenylacetyl)amino]-1,2-thiazol-2-yl]but-3-enoate
CAS
34104-24-0;94822-51-2
化学式
C17H18N2O4S
mdl
——
分子量
346.407
InChiKey
RBADMFFHAZNMIX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:24
-
可旋转键数:7
-
环数:2.0
-
sp3杂化的碳原子比例:0.24
-
拓扑面积:101
-
氢给体数:1
-
氢受体数:5
反应信息
-
作为反应物:参考文献:名称:青霉素的转化。第一部分。6β-苯基乙酰胺基半胱氨酸亚砜的制备和重排摘要:已经制备了6β-苯基乙酰胺基二十二烷酸酯和N-叔丁基酰胺的异构亚砜。(R)-亚砜可以被热转化为(S)-异构体。氘标记表明异构化是通过亚硫酸中间体进行的。亚砜与乙酸酐的重排主要产生相应的乙酰氧基青am和乙酰氧基头孢菌素。乙酰氧基取代的戊烷的两种异构体可以通过适当的亚砜的异构化形成。DOI:10.1039/j39710003540
-
作为产物:参考文献:名称:青霉素的转化。第一部分。6β-苯基乙酰胺基半胱氨酸亚砜的制备和重排摘要:已经制备了6β-苯基乙酰胺基二十二烷酸酯和N-叔丁基酰胺的异构亚砜。(R)-亚砜可以被热转化为(S)-异构体。氘标记表明异构化是通过亚硫酸中间体进行的。亚砜与乙酸酐的重排主要产生相应的乙酰氧基青am和乙酰氧基头孢菌素。乙酰氧基取代的戊烷的两种异构体可以通过适当的亚砜的异构化形成。DOI:10.1039/j39710003540
文献信息
-
SAKO M.; MAKI Y., CHEM. AND PHARM. BULL., 1978, 26, NO 4, 1236-1239作者:SAKO M.、 MAKI Y.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
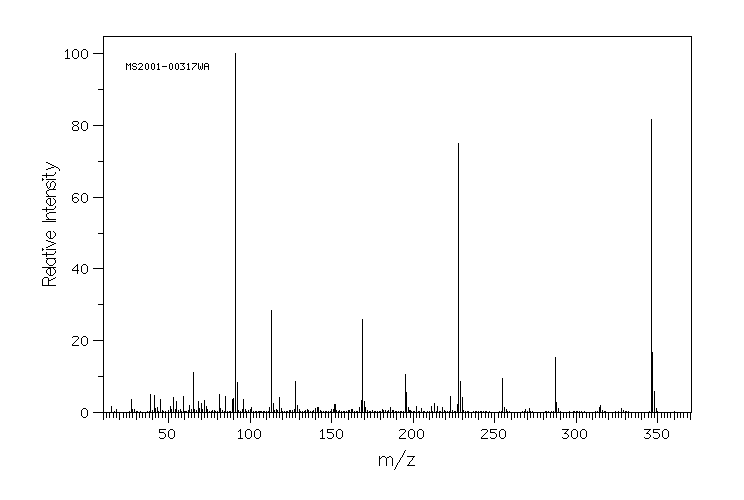
质谱MS
-
碳谱13CNMR
-
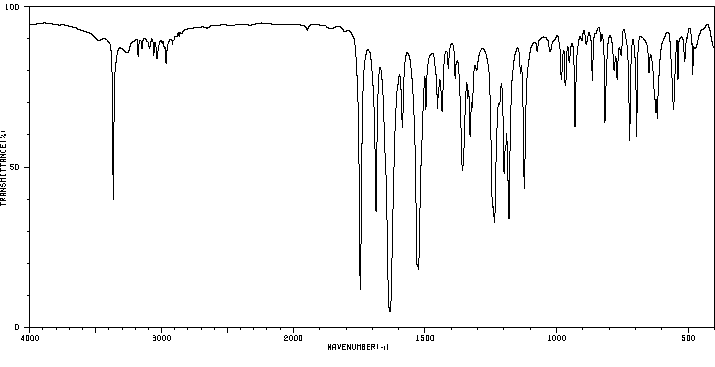
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸