(E)-1-fluoro-4-(2-nitroprop-1-en-1-yl)benzene | 775-31-5
中文名称
——
中文别名
——
英文名称
(E)-1-fluoro-4-(2-nitroprop-1-en-1-yl)benzene
英文别名
(E)-1-fluoro-4-(2-nitroprop-1-enyl)benzene;Benzene, 1-fluoro-4-(2-nitropropenyl)-;1-fluoro-4-[(E)-2-nitroprop-1-enyl]benzene
CAS
775-31-5
化学式
C9H8FNO2
mdl
——
分子量
181.166
InChiKey
VOAXWARMFBBINZ-VOTSOKGWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:64-66 °C
-
沸点:262.2±15.0 °C(Predicted)
-
密度:1.230±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:3
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : 4-Fluoro-Beta-Nitropropenylbenzene
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, Oral (Category 3), H301
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
T Toxic R25
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Danger
Hazard statement(s)
Toxic if swallowed.
Precautionary statement(s)
P301 + P310 IF SWALLOWED: Immediately call a POISON CENTER or doctor/
physician.
Supplemental Hazard none
Statements
Other hazards
This substance/mixture contains no components considered to be either persistent, bioaccumulative and
toxic (PBT), or very persistent and very bioaccumulative (vPvB) at levels of 0.1% or higher.
SECTION 3: Composition/information on ingredients
Substances
Molecular weight : 181,17 g/mol
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
4-Fluoro-Beta-Nitropropenylbenzene
Acute Tox. 3; H301 <= 100 %
Hazardous ingredients according to Directive 1999/45/EC
Component Classification Concentration
4-Fluoro-Beta-Nitropropenylbenzene
T, R25 <= 100 %
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Take victim immediately to hospital. Consult a physician.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
No data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Nature of decomposition products not known.
Advice for firefighters
Wear self-contained breathing apparatus for firefighting if necessary.
Further information
No data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Wear respiratory protection. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
For personal protection see section 8.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Storage class (TRGS 510): Non-combustible, acute toxic Cat.3 / toxic hazardous materials or hazardous
materials causing chronic effects
Specific end use(s)
Apart from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Avoid contact with skin, eyes and clothing. Wash hands before breaks and immediately after handling
the product.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face particle
respirator type N99 (US) or type P2 (EN 143) respirator cartridges as a backup to engineering
controls. If the respirator is the sole means of protection, use a full-face supplied air respirator. Use
respirators and components tested and approved under appropriate government standards such
as NIOSH (US) or CEN (EU).
Control of environmental exposure
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour No data available
c) Odour Threshold No data available
d) pH No data available
e) Melting point/freezing No data available
point
f) Initial boiling point and No data available
boiling range
g) Flash point No data available
h) Evaporation rate No data available
i) Flammability (solid, gas) No data available
j) Upper/lower No data available
flammability or
explosive limits
k) Vapour pressure No data available
l) Vapour density No data available
m) Relative density No data available
n) Water solubility No data available
o) Partition coefficient: n- No data available
octanol/water
p) Auto-ignition No data available
temperature
q) Decomposition No data available
temperature
r) Viscosity No data available
s) Explosive properties No data available
t) Oxidizing properties No data available
Other safety information
No data available
SECTION 10: Stability and reactivity
Reactivity
No data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
No data available
Conditions to avoid
No data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
No data available
Skin corrosion/irritation
No data available
Serious eye damage/eye irritation
No data available
Respiratory or skin sensitisation
No data available
Germ cell mutagenicity
No data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
No data available
Specific target organ toxicity - single exposure
No data available
Specific target organ toxicity - repeated exposure
No data available
Aspiration hazard
No data available
Additional Information
RTECS: Not available
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
No data available
Persistence and degradability
No data available
Bioaccumulative potential
No data available
Mobility in soil
No data available
Results of PBT and vPvB assessment
This substance/mixture contains no components considered to be either persistent, bioaccumulative and
toxic (PBT), or very persistent and very bioaccumulative (vPvB) at levels of 0.1% or higher.
Other adverse effects
No data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: 2811 IMDG: 2811 IATA: 2811
UN proper shipping name
ADR/RID: TOXIC SOLID, ORGANIC, N.O.S. (4-Fluoro-Beta-Nitropropenylbenzene)
IMDG: TOXIC SOLID, ORGANIC, N.O.S. (4-Fluoro-Beta-Nitropropenylbenzene)
IATA: Toxic solid, organic, n.o.s. (4-Fluoro-Beta-Nitropropenylbenzene)
Transport hazard class(es)
ADR/RID: 6.1 IMDG: 6.1 IATA: 6.1
Packaging group
ADR/RID: III IMDG: III IATA: III
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
No data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
No data available
Chemical Safety Assessment
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-N-(1-(4-fluorophenyl)prop-1-en-2-yl)acetamide 1435940-38-7 C11H12FNO 193.221
反应信息
-
作为反应物:描述:(E)-1-fluoro-4-(2-nitroprop-1-en-1-yl)benzene 在 双氧水 、 sodium hydroxide 作用下, 以 甲醇 、 水 为溶剂, 反应 0.17h, 以78%的产率得到3-(4-fluorophenyl)-2-methyl-2-nitrooxirane参考文献:名称:Nitroepoxides as Versatile Precursors to 1,4-Diamino Heterocycles摘要:Nitroepoxides are easily transformed into 1,4-diamino heterocycles such as quinoxalines and pyrazines by treatment with 1,2-benzenediamines and ammonia, respectively. Additionally, related saturated heterocycles, such as piperazines and tetrahydroquinoxalines, can be accessed by treatment with 1,2-diamines and a reducing agent. These transformations are efficient, provide access privileged, bioactive structures, and produce minimal waste.DOI:10.1021/ol500444z
-
作为产物:描述:参考文献:名称:具有深手性口袋的磷配体的设计:通过不对称氢化实际合成手性β-芳胺摘要:WingPhos是一种C 2对称的双磷配体,具有较深且定义明确的手性口袋。在(E)-β-芳基-N-乙酰基酰胺,环状β-芳基酰胺和杂环β-芳基酰胺的铑催化不对称氢化反应中显示出很高的效率。可以合成一系列具有良好ee值(nbd = 3,5-降冰片二烯; TON =周转数)的手性β-芳基异丙胺,2-氨基四氢呋喃和3-氨基苯并二氢吡喃。DOI:10.1002/anie.201300646
文献信息
-
[EN] FLUOROMETHYL-SUBSTITUTED PYRROLE CARBOXAMIDES<br/>[FR] PYRROLE CARBOXAMIDES SUBSTITUÉS PAR UN FLUOROMÉTHYLE申请人:GRUENENTHAL GMBH公开号:WO2014032801A1公开(公告)日:2014-03-06The invention relates to pyrrole carboxamides bearing a fluoromethyl- moiety as voltage gated calcium channel blockers, to pharmaceutical compositions containing these compounds and also to these compounds for use in the treatment and/or prophylaxis of pain and further diseases and/or disorders.
-
FLUOROMETHYL-SUBSTITUTED PYRROLE CARBOXAMIDES申请人:GRUNENTHAL GMBH公开号:US20140066426A1公开(公告)日:2014-03-06The invention relates to pyrrole carboxamides bearing a fluoromethyl-moiety as voltage gated calcium channel blockers, to pharmaceutical compositions containing these compounds and also to these compounds for use in the treatment and/or prophylaxis of pain and further diseases and/or disorders.
-
A Novel Entry to 3,4,5-Trisubstituted 2-Pyrrolidones from Isoxazoline-N-oxides作者:Alexey Sukhorukov、Petr Zhmurov、Pavel Ushakov、Roman Novikov、Sema IoffeDOI:10.1055/s-0037-1610213日期:2018.9A novel strategy for the synthesis of stereochemically defined 3,4,5-trisubstituted 2-pyrrolidones was developed. The suggested approach involves reductive domino-type recyclization of 3-aminomethyl-substituted isoxazolines as a key stage. The latter are prepared via α-C–H functionalization of readily available isoxazoline-N-oxides.
-
Synthesis, Antiproliferative and Pro-Apoptotic Effects of Nitrostyrenes and Related Compounds in Burkitt's Lymphoma作者:Andrew J. Byrne、Sandra A. Bright、Darren Fayne、James P. McKeown、Thomas McCabe、Brendan Twamley、Clive Williams、Mary J. MeeganDOI:10.2174/1573406413666171002123907日期:2018.2.6identified as a lead target structure for the development of particularly effective compounds targeting Burkitt's lymphoma (BL). OBJECTIVES The aims of the curent study were to synthesise a panel of nitrostyrene compounds and to evaluate their activity in Burkitt's lymphoma (BL). METHODS A panel of structurally varied compounds were designed and synthesised using Henry Knoevenagel condensation reactions.背景技术淋巴细胞癌(淋巴瘤)约占全世界恶性疾病的12%。硝基苯乙烯支架被认为是开发针对Burkitt淋巴瘤(BL)的特别有效的化合物的主要靶标结构。目的目前的研究目的是合成一组硝基苯乙烯化合物并评估其在伯基特氏淋巴瘤(BL)中的活性。方法使用Henry Knoevenagel缩合反应设计和合成一组结构变化的化合物。单晶X射线分析证实了这些新颖结构的六个实例的E构型。还研究了许多与硝基苯乙烯有关的化合物,包括1,3-双(芳基)-2-硝基丙烯与含有硝基乙烯基药效团的杂环支架,例如3-硝基-2-苯基-2H-色烯。使用BL细胞系EBV-MUTU-1和EBV + DG-75(耐化学性)评估化合物的抗增殖活性,以建立初步的结构活性关系。结果成功建立了具有优化的硝基苯乙烯骨架和3-硝基-2-苯基-2Hchromne结构的铅化合物,在MUTU-1细胞中的典型IC50值分别为0.45 µM和0.47 µM,
-
Highly regio- and diastereoselective [3 + 2]-cycloadditions involving indolediones and α,β-disubstituted nitroethylenes作者:Madhuri P. Rao、Shubha S. Gunaga、Johannes Zuegg、Rambabu Pamarthi、Madhu GaneshDOI:10.1039/c9ob01429b日期:——A highly diastereoselective [3 + 2]-cycloaddition strategy involving multiple oxindoles and several α,β-disubstituted nitroethylenes is developed to access tetra-substituted α-spiropyrrolidine frameworks. A variety of α-amino acids were employed for the first time in order to generate azomethine ylides under thermal conditions, affording regioisomers 13 and 14 merely by changing the α-substituents一种高度非对映选择性的[3 + 2]-环加成策略涉及多个羟吲哚和几个α,β-二取代的硝基乙烯被开发来访问四取代的α-螺并吡咯烷骨架。为了在热条件下生成甲亚胺基团,首次使用了多种α-氨基酸,仅通过改变α-氨基酸的α-取代基(R = H和取代的碳)即可提供区域异构体13和14。该反应可耐受甘氨酸,羟吲哚和硝基乙烯上的各种空间需求,富电子和缺电子的芳基和氮取代基。遵循绿色化学原理,操作简单,例如使用无金属,非惰性的环境,使用非卤代溶剂和易于分离,使此过程对扩大机会具有吸引力。该反应具有良好的产率(80-94%)和非对映选择性(高达98:2),有利于(顺式,顺式)-螺氧并吲哚,在相同条件下与β-硝基苯乙烯相比,区域选择性相反。两种带有未保护酰胺的螺吡咯烷环加合物对革兰氏阳性MRSA表现出显着活性。
表征谱图
-
氢谱1HNMR
-
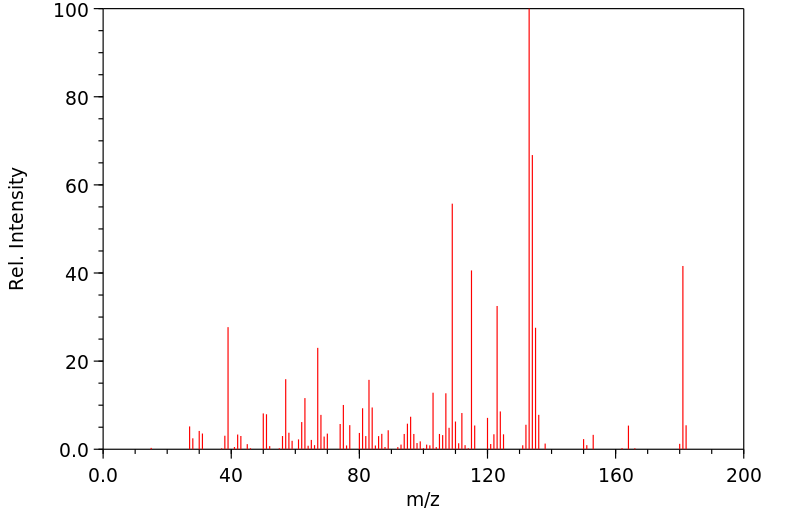
质谱MS
-
碳谱13CNMR
-
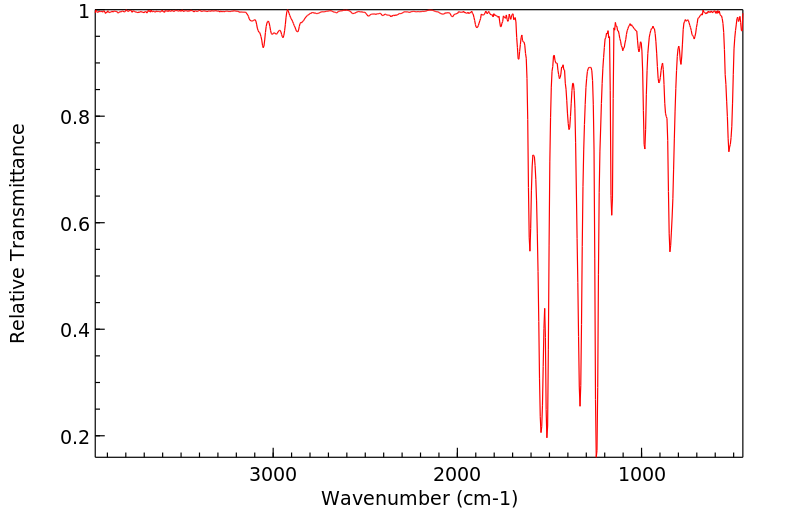
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫