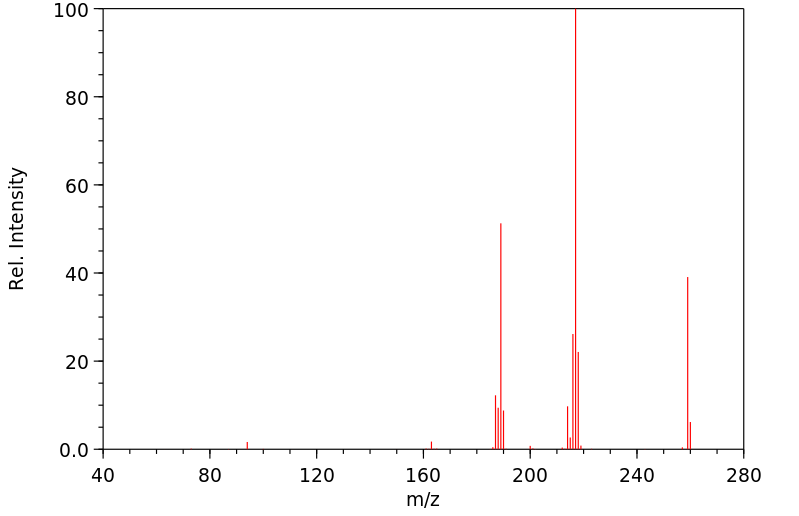
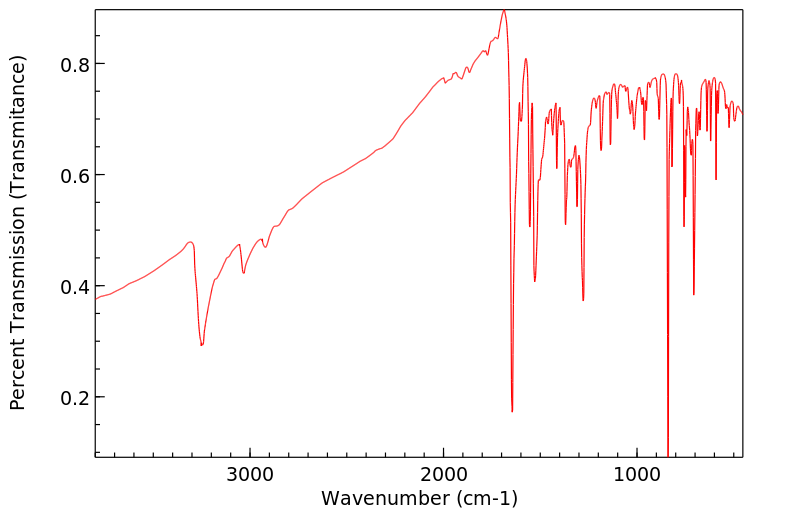
N-(1-pyrenyl)acetamide | 22755-15-3
中文名称
——
中文别名
——
英文名称
N-(1-pyrenyl)acetamide
英文别名
N-Acetyl-1-aminopyrene;N-pyren-1-ylacetamide;1-Acetylaminopyrene;N-acetylpyreneamine;1-Acetamidopyrene;N-pyren-1-yl-acetamide
CAS
22755-15-3
化学式
C18H13NO
mdl
——
分子量
259.307
InChiKey
GJNGMQYHOZFQNX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:20
-
可旋转键数:1
-
环数:4.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2924299090
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 1-ACETAMIDOPYRENE
CAS-No. : 22755-15-3
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
Label elements
Caution - substance not yet tested completely.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C18H13NO
Molecular Weight : 259,31 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx)
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Avoid
breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Choose body protection in relation to its type, to the concentration and amount of dangerous
substances, and to the specific work-place., The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection is not required. Where protection from nuisance levels of dusts are desired,
use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing 260 - 261 °C
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 4,184
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation
May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: 3077 IMDG: 3077 IATA: 3077
UN proper shipping name
ADR/RID: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (1-Acetamidopyrene)
IMDG: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (1-Acetamidopyrene)
IATA: Environmentally hazardous substance, solid, n.o.s. (1-Acetamidopyrene)
Transport hazard class(es)
ADR/RID: 9 IMDG: 9 IATA: 9
Packaging group
ADR/RID: III IMDG: III IATA: III
Environmental hazards
ADR/RID: yes IMDG Marine pollutant: yes IATA: yes
Special precautions for user
Further information
EHS-Mark required (ADR 2.2.9.1.10, IMDG code 2.10.3) for single packagings and combination
packagings containing inner packagings with Dangerous Goods > 5L for liquids or > 5kg for solids.
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Role of O-acetyltransferase in activation of oxidised metabolites of the genotoxic environmental pollutant 1-nitropyrene摘要:The genotoxic environmental contaminant l-nitropyrene is metabolised in mammalian systems by pathways more complex than the straightforward nitroreduction which accounts for most of its biological activity in bacteria. In order to evaluate the role of O-acetyltransferase (OAT) activity in generation of genotoxic intermediates from 1-nitropyrene, the mutagenicity of the major primary oxidised metabolites of 1-nitropyrene was characterised in the Ames Salmonella typhimurium plate incorporation assay with strain TA98, and with variants of TA98 deficient (TA98/1,8-DNP6) or enhanced (YG1024) in O-acetyltransferase. 1-Nitropyren-3-ol was more mutagenic in the absence than in the presence of S9, while 1-nitropyren-4-ol, 1-nitropyren-6-ol and 1-nitropyren-8-ol required S9 for maximum expression of mutagenicity. 1-Nitropyren-4-ol (176 rev/nmol without S9, 467 rev/nmol with S9 in TA98) and 1-nitropyren-6-ol (13 rev/nmol without S9, 266 rev/nmol with S9 in TA98) were overall the most potent nitropyrenol isomers assayed. 1-Acetamidopyren-8-ol and 2-acetamidopyrene 4,5-quinone were only minimally active. 1-Acetamidopyren-3-ol exhibited direct-acting mutagenicity. 1-Acetamidopyren-6-ol, previously shown to be a major contributor to mutagenicity in the urines of rats dosed with l-nitropyrene (Ball et al., 1984b), was confirmed as a potent (359 rev/nmol) S9-dependent mutagen. Both the direct-acting and the S9-dependent mutagenicity of all the compounds studied was enhanced in the OAT-overproducing strain and much diminished (though not always entirely lost) in the OAT-deficient strain, showing that OAT amplifies expression of the genotoxicity of these compounds. 1-Acetamidopyren-6-ol required both 89 and OAT activity in order to exhibit any mutagenicity; this finding strongly implicates N-hydroxylation followed by O-esterification, as opposed to further S9-catalyzed ring oxidation, as a major route of activation for urinary metabolites of 1-nitropyrene.DOI:10.1016/s0165-1218(96)90026-9
-
作为产物:描述:参考文献:名称:Vollmann et al., Justus Liebigs Annalen der Chemie, 1937, vol. 531, p. 1,109摘要:DOI:
文献信息
-
<i>N</i>-Porphyrinylamino and -amido Compounds by Addition of an Amino or Amido Nitrogen to a Porphyrin Meso Position作者:K. Jayaraj、A. Gold、L. M. Ball、P. S. WhiteDOI:10.1021/ic000112r日期:2000.8.1however, allowed the aryl plane to rotate toward coplanarity with the porphyrin plane, resulting in conjugation of the highest occupied aryl and porphyrin molecular orbitals through the nitrogen lone pair. In developing routes to the amino-linked compounds, the facile formation of fused azaaryl chlorins via an oxidative intramolecular cycloaddition was observed. An aryl carbon ortho to the meso linkage该报告描述了一系列八乙基卟啉衍生物的合成和表征,其中卟啉pi网络通过内消旋氨基或酰胺基氮与苯基,3-荧蒽基或1-吡啶基芳族系统连接。已获得不含金属的碱以及锌(II)和铁(III)配合物。这些化合物代表了卟啉和延伸的pi网络之间通过直接连接到卟啉介孔位置的氮原子进行连接的第一个例子。对无金属的碱和锌络合物的1 H NMR研究表明,在酰胺基连接的加合物中,含有芳基取代基的平面垂直于卟啉平面。但是,通过仲氨基氮原子的连接使芳基平面向与卟啉平面共面旋转,导致通过氮孤对共轭占据最高的芳基和卟啉分子轨道。在发展成与氨基连接的化合物的途径中,观察到通过氧化性分子内环加成法容易形成稠合的氮杂芳基二氢卟酚。与内消旋键邻位的芳基碳攻击相邻的吡咯环的β-碳,并伴随着吡咯β-乙基取代基的1,2-迁移和最初形成的大环的二电子氧化。所得结构类似于苯二甲酰氯。不含金属的碱的电子光谱的特征在于在可见光区域中存在强烈的长波
-
Synthesis and Photophysics of a 1-Pyrenyl Substituted 2‘-Deoxyuridine-5-Carboxamide Nucleoside: Electron Transfer Products as CIS INDO/S Excited States作者:Charles E. Kerr、C. Denise Mitchell、Jeb Headrick、Bruce E. Eaton、Thomas L. NetzelDOI:10.1021/jp992773j日期:2000.2.1This paper reports results of the synthesis and photophysical study of 5-(N-carboxy-1-aminopyrenyl)-2‘-deoxyuridine (PA-dU) and its spectroscopic model N-acetyl-1-aminopyrene (PA-Ac). Absorbance and emission spectra, emission quantum yields, and emission lifetimes are reported for both compounds in three solvents. The data show that the emission yield quenching of PA-dU relative to PA-Ac varies from本文报道了 5-(N-carboxy-1-aminopyrenyl)-2'-deoxyuridine (PA-dU) 及其光谱模型 N-acetyl-1-aminopyrene (PA-Ac) 的合成和光物理研究结果。报告了两种化合物在三种溶剂中的吸光度和发射光谱、发射量子产率和发射寿命。数据显示,在溶剂系列 THF、MeCN 和 MeOH 中,PA-dU 相对于 PA-Ac 的发射产率猝灭在 95% 到 99% 之间变化。与 PA-Ac 的 (π,π*)1 发射的单指数动力学相反,PA-dU 核苷的 (π,π*)1 发射在 THF 中衰减两个寿命,在 MeCN 和 MeOH 中衰减三个。多指数发射衰减可能是由于存在多个核苷构象异构体。PA-dU 在 THF、MeCN 和 MeOH 中的平均 (π,π*)1 寿命分别为 4.8、2.7 和 0。55 ns,分别对应于 58、81 和 96%
-
N-酰基芘胺的制备方法以及1-羟基芘的制备方法申请人:江苏广域化学有限公司公开号:CN112679377B公开(公告)日:2022-09-20
-
Novel additives for imparting mar and scratch resistance and compositions comprising the same申请人:——公开号:US20040116585A1公开(公告)日:2004-06-17Additives comprising dispersed silica nanoparticles are disclosed. The silica nanoparticles are dispersed in an aminoplast, such as a modified aminoplast formed from the reaction between an aminoplast, a modifying component, and optionally a siloxane. The resulting additives can be added to coating compositions to provide improved mar and/or scratch resistance.
-
Novel additives for imparting Mar and scratch resistance and compositions comprising the same申请人:Ambrose R. Ronald公开号:US20060078748A1公开(公告)日:2006-04-13Additives comprising dispersed silica nanoparticles are disclosed. The silica nanoparticles are dispersed in an aminoplast, such as a modified aminoplast formed from the reaction between an aminoplast, a modifying component, and optionally a siloxane. The resulting additives can be added to coating compositions to provide improved mar and/or scratch resistance.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-1,2-二(1-芘基)环丁烷
顺式-1,2-二(1-芘基)环丁烷
顺式-(-)-苯并(a)芘-7,8-二醇-9,10-环氧化物
雄甾烷
还原黑29
还原黄4
还原金橙G
还原绿2
还原绿1
还原紫3B
还原紫 10
还原深蓝BO
还原橙4
还原橙2
还原兰黑BBN
还原亮橙IRK
试剂N1,N1,N3,N3,N6,N6,N8,N8-Octakis(4-methoxyphenyl)-1,3,6,8-pyrenetetramine
蒽酮紫79
蒽缔蒽酮
蒽并(1,2,3,4-ghi)苝
蒽嵌蒽
蒽[9,1,2-cde]苯并[rst]戊芬
萘并[2'.8',2.4]晕苯
萘并[2',1',8',7':4,10,5]蒽并[1,9,8-abcd]晕苯
萘并[1,8-gh:4,5-g'h']二喹啉
萘并(8,1,2-bcd)苝
萘并(2,3-a)晕苯
萘并(2,1,8-qra)萘并萘-7 12-二酮
萘并(1,2,3-mno)醋菲烯
萘[2,3-a]芘
菲并[1,10,9,8-opqra]苝
茚并(1,2,3-cd)芘
苯胺,2-氯-3-(苯基甲氧基)-
苯并[xyz]庚芬
苯并[wx]萘并[2,1,8,7-hijk]庚省
苯并[rst]菲并[1,10,9-cde]戊芬
苯并[rst]戊酚-5-甲醛
苯并[pqr]四苯-5-基甲酸根
苯并[pqr]四苯-11-基甲酸根
苯并[pqr]二萘并[8,1,2-bcd:2',1',8'-lmn]苝
苯并[p]萘并[1,8,7-ghi]屈
苯并[l]芘-8-醇
苯并[ghi]苝
苯并[e]芘
苯并[b]芘-6-基甲醇
苯并[b]芘-6,12-二酮
苯并[b]芘-3,6-二酮
苯并[b]芘-1,6-二酮
苯并[a]芘-9,10-环氧化物
苯并[a]芘-7-醇