4-benzylidene-2-methyl-5-oxazolone | 143417-47-4
中文名称
——
中文别名
——
英文名称
4-benzylidene-2-methyl-5-oxazolone
英文别名
(E)-4-benzylidene-2-methyloxazol-5(4H)-one;2-Methyl-4-(phenylmethylene)oxazol-5(4H)-one;(4E)-4-benzylidene-2-methyl-1,3-oxazol-5-one
CAS
143417-47-4
化学式
C11H9NO2
mdl
——
分子量
187.198
InChiKey
BWQBTJRPSDVWIR-JXMROGBWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:38.7
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (Z)-4-亚苄基-2-甲基噁唑-5(4h)-酮 (Z)-4-benzylidene-2-methyl-5(4H)-oxazolone 38879-46-8 C11H9NO2 187.198 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (Z)-4-亚苄基-2-甲基噁唑-5(4h)-酮 (Z)-4-benzylidene-2-methyl-5(4H)-oxazolone 38879-46-8 C11H9NO2 187.198
反应信息
-
作为反应物:描述:参考文献:名称:碳酸酐酶XII抑制剂克服了成胶质细胞瘤的替莫唑胺耐药性。摘要:天然产物初级磺酰胺,psammaplin C(1)与临床使用的化疗药物(包括替莫唑胺)组合使用时,可以逆转多药耐药性并增加成胶质母细胞瘤(一种高度侵袭性原发性脑肿瘤)的存活率。我们以前表明1的作用机理是新颖的,其作用是由于碳酸酐酶XII(CA XII)抑制而间接干扰P-糖蛋白药物外排活性。为了建立构效关系,设计,合成了1的45个衍生物,并针对一组CA同工型进行了评估。化合物55被确定为CA XII的有效抑制剂(Ki = 0.56 nM),并使用胶质母细胞瘤患者的样品进行了体内和体外研究。DOI:10.1021/acs.jmedchem.9b00282
-
作为产物:描述:(Z)-4-亚苄基-2-甲基噁唑-5(4h)-酮 以 乙腈 为溶剂, 以20%的产率得到4-benzylidene-2-methyl-5-oxazolone参考文献:名称:Benzylidene-Oxazolones as Molecular Photoswitches摘要:The synthesis and photochemical study of a family of molecular switches inspired by the green fluorescent protein (UP) chromophore is presented. These compounds can be easily synthesized, and their photophysical properties may be tuned. Due to their efficient photoisomerization and high stability, these compounds can be switched on/off by using light and heat or light with different wavelengths.DOI:10.1021/ol301741g
文献信息
-
METHOD OF SCREENING FOR AGENTS FOR DIFFERENTIATING STEM CELLS申请人:MINERVA BIOTECHNOLOGIES CORPORATION公开号:US20180263964A1公开(公告)日:2018-09-20The present application discloses a method for identifying an agent for the treatment or prevention of cancer or metastatic cancer comprising the steps of contacting stem cell with a potential agent, and identifying an agent that induces differentiation, or inhibits stem cell pluripotency or growth of the stem cell, wherein such agent is determined to be an anti-cancer agent.本申请公开了一种用于识别治疗或预防癌症或转移性癌症的药剂的方法,包括以下步骤:将干细胞与潜在药剂接触,并识别诱导分化的药剂,或抑制干细胞多能性或干细胞生长的药剂,其中这种药剂被确定为抗癌药剂。
-
Reaction of 4-benzylidene-2-methyl-5-oxazolone with amines, Part 2: Influence of substituents in para-position in the phenyl ring and a substituent on amine nitrogen atom on the reaction kinetics作者:B. Bet?akowska、B. Banecki、C. Czaplewski、L. ?ankiewicz、W. WiczkDOI:10.1002/kin.10039日期:2002.3ring-opening reaction was studied. The substituents (OH, OCH3, N(CH3)2, Cl, NO2) in para-position of the phenyl ring of Ox substantially modified the rate of the reaction with benzylamine in acetonitrile. The rate of the Ox ring-opening reaction decreased with increase of the electron-donating properties of the substituent. A good correlation between the rate constants of the reaction of 4-(4′-sub胺(苄胺、N-甲基-苄胺、N-异丙基-苄胺、N-甲基-丁胺、N-乙基-丁胺、仲丁胺和叔丁胺)的结构对速率常数的影响研究了4-亚苄基-2-甲基-5-恶唑酮(Ox)的开环反应。速率常数的对数与查顿的空间取代基常数 ν 之间的良好相关性以及与简单支化方程形式的良好相关性表明,由于亲核试剂的 C1 碳原子上的取代降低了反应速率,因此存在空间效应。另外,研究了Ox的亚苄基部分的结构对恶唑酮开环反应速率的影响。取代基(OH、O 、N(CH3)2、Cl、NO2) 在 Ox 的苯环的对位上显着改变了与苄胺在乙腈中的反应速率。Ox开环反应的速率随着取代基给电子性质的增加而降低。4-(4'-取代的亚苄基)-2-甲基-5-恶唑酮与苄胺反应的速率常数与反应中心的电子密度(恶唑酮环的 C5 碳)之间具有良好的相关性,计算公式为建立了从头算法,建立了Hammett取代基常数和CR方程。© 2002 Wiley Periodicals
-
[EN] CARBONIC ANHYDRASE INHIBITORS<br/>[FR] INHIBITEURS D'ANHYDRASE CARBONIQUE申请人:UNIV GRIFFITH公开号:WO2018204987A1公开(公告)日:2018-11-15Compounds are provided which are inhibitors of the CAXII enzyme. Due to the interaction between CAXII and Pgp, such compounds may be useful in lowering the chemoresistance of a cancer allowing for co-administration with existing anti-cancer agents.提供了抑制CAXII酶的化合物。由于CAXII和Pgp之间的相互作用,这些化合物可能有助于降低癌症的化疗抗药性,从而可以与现有的抗癌药物同时使用。
-
Thiourea to bicyclic scaffolds: highly regio- and stereoselective routes to dithiazolopyrimidines作者:Lal Dhar S. Yadav、Vijai K. RaiDOI:10.1016/j.tet.2007.04.033日期:2007.7Microwave-activated solvent-free Michael addition of 3-imino-1,4,2-dithiazoles to 4-arylidene-5(4H)-oxazolones furnished isolable adducts regio- and diastereoselectively, which underwent ring transformation to yield the target dithiazolopyrimidines. Alternatively, the similar conjugate addition of methanesulfinylmethylisothioureas to 4-arylidene-5(4H)-oxazolones diastereoselectively afforded Michael
-
Process for the preparation of n-acetylphenylalanine申请人:Hoechst Aktiengesellschaft公开号:US04892971A1公开(公告)日:1990-01-09In the process for the preparation of N-acetylphenylalanine by opening the ring of 2-methyl-4-benzylidene-1,3-oxazolin-5-one with water to give 2-acetaminocinnamic acid and subsequently catalytically hydrogenating the latter, both reaction stages are carried out in a mixture of an aliphatic C.sub.3 -ketone to C.sub.10 -ketone or a water-miscible ether and water as the solvent, and the hydrogenation of the 2-acetaminocinnamic acid is carried out at temperatures of 10.degree. to 50.degree. C. and pressures of 1 to 15 bar in the presence of a supported palladium catalyst.
表征谱图
-
氢谱1HNMR
-
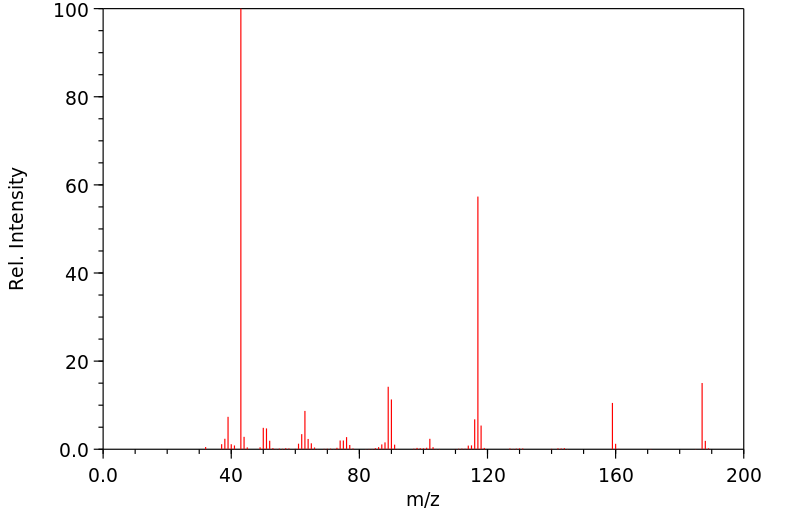
质谱MS
-
碳谱13CNMR
-
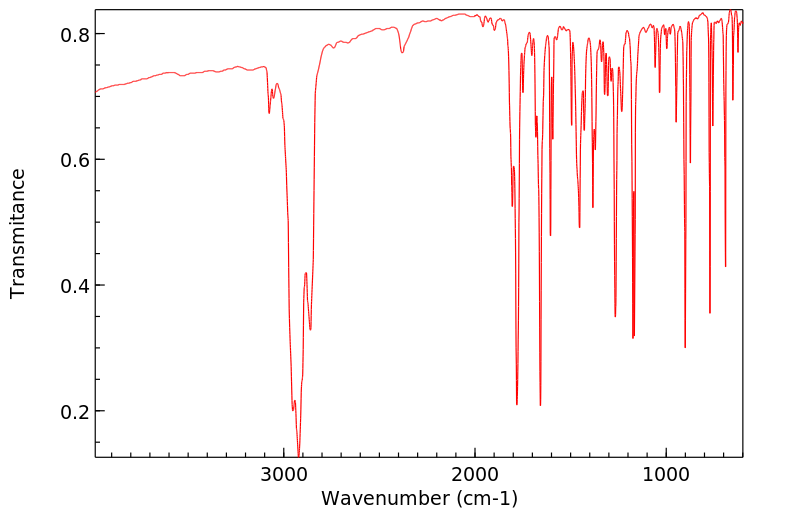
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸